Peanut Coffee: Enhancement of Nutritional, Physicochemical, and Sensory Characteristics in Coffee Brewed with Conventional and High-Oleic Peanut Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Nutritional Characteristics
2.3. Physicochemical Characteristics
2.4. Sensory Characteristics
2.5. Statistical Analysis
3. Results and Discussion
3.1. Nutritional Characteristics
3.2. Physicochemical Characteristics
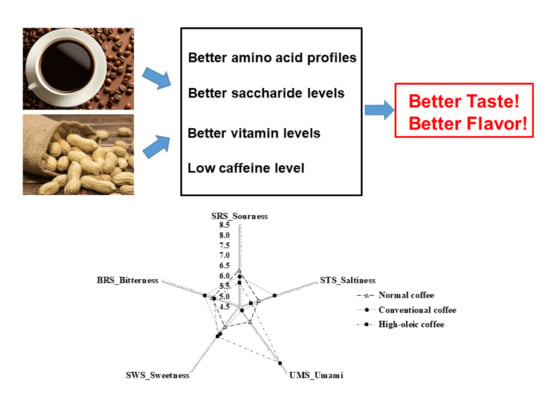
3.3. Sensory Characteristics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Choi, K.P.; Chae, D.J.; Ryoo, J.E. Trends of coffee industry and prospect in Korea. Food Ind. Nutr. 2014, 19, 1–4. [Google Scholar]
- Yang, N.; Liu, C.; Degn, T.K.; Munchow, M.; Fisk, I. Determination of volatile marker compounds of common coffee roast defects. Food Chem. 2016, 211, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Kim, H.S.; Hong, S.J.; Cho, J.J.; Lee, J.; Shin, E.C. Comparison of the retention rates of thiamin, riboflavin, and niacin between normal and high-oleic peanuts after roasting. Appl. Biol. Chem. 2018, 61, 449–458. [Google Scholar] [CrossRef]
- Kim, J.K.; Kwon, S.J.; Kang, H.I.; Lee, J.H.; Kang, J.S.; Seo, K.I. Quality characteristics and antioxidant effects of peanut sprout soybean yogurt. Korea Soc. Food Preserv. 2013, 20, 199–206. [Google Scholar] [CrossRef]
- Moon, J.K.; Shibamoto, T. Role of roasting conditions in the profile of volatile flavor chemicals formed from coffee beans. J. Agric. Food Chem. 2009, 50, 1529–1534. [Google Scholar] [CrossRef]
- Lee, M.J.; Kim, S.H.; Kim, J.H.; Lee, S.W.; Yeom, D.M. A study of coffee bean characteristics and coffee flavors in relation to roasting. Food Sci. Nutr. 2013, 42, 255–261. [Google Scholar]
- Caporaso, N.; Whitwort, M.B.; Cui, C.; Fisk, I.D. Variability of single bean coffee volatile compounds of Arabica and Robusta roasted coffees analyzed by SPME-GC-MS. Food Res. Int. 2018, 108, 628–640. [Google Scholar] [CrossRef]
- Kim, J.S.; Jung, H.Y.; Park, E.Y.; Noh, B.S. Flavor analysis of commercial Korean distilled spirits using an electronic nose and electronic tongue. J. Food Sci. Technol. 2016, 48, 117–121. [Google Scholar]
- Dong, W.J.; Hu, R.; Long, R.; Li, H.; Zhang, Y.; Zhu, K. Comparative evaluation of the volatile profiles and taste properties of roasted coffee beans as affected by drying method and detected by electronic nose, electronic tongue, and HS-SPME-GC-MS. Food Chem. 2019, 272, 723–731. [Google Scholar] [CrossRef]
- Morais, T.C.; Rodrigues, D.R.; Souto, U.T.; Lemos, S.G. A simple voltammetric electronic tongue for the analysis of coffee adulterations. Food Chem. 2019, 273, 31–38. [Google Scholar] [CrossRef]
- Yang, S.I.; Kim, H.J.; Yang, Y.S.; Oh, B.S.; Kim, D.C. Comparison of antioxidative ability between coffee bean and coffee residue extracts. Food Serv. Ind. Manag. 2014, 10, 73–82. [Google Scholar]
- Liu, C.; Yang, N.; Yang, Q.; Ayed, C.; Linforth, R.; Fisk, I.D. Enhancing Robusta coffee aroma by modifying flavor precursors in the green coffee bean. Food Chem. 2019, 281, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, K.; Shibamoto, T. Chlorogenic acid and caffeine contents in various commercial brewed coffees. Food Chem. 2008, 106, 217–221. [Google Scholar] [CrossRef]
- Kim, S.E.; Kim, J.H.; Lee, S.W.; Lee, M.J. A study of roasting conditions on benzo[a]pyrene content in coffee beans. Food Sci. Nutr. 2013, 42, 134–138. [Google Scholar]
- Lee, B.E.; Lee, H.J.; Cho, E.A.; Hwang, K.T. Fatty acid compositions of fats in commercial coffee creamers and instant coffee mixes and their sensory characteristics. Food Sci. Nutr. 2012, 41, 362–368. [Google Scholar]
- Kim, D.S.; Kim, H.S.; Lee, J.; Pan, J.H.; Kim, Y.J.; Kim, J.K.; Woo, S.M.; Shin, E.C. Wasabia koreana Nakai: A preliminary study on nutrients and chemical compounds that may impact sensory properties. Molecules 2018, 23, 2512. [Google Scholar] [CrossRef] [Green Version]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Folin, O.; Danis, W. On phosphotungstic-phosphomolybdic compounds as color reagents. J. Biol. Chem. 1912, 12, 239–243. [Google Scholar]
- Lee, J.; Cho, J.J.; Hong, S.J.; Kim, D.S.; Boo, C.G.; Shin, E.C. Platycodon grandiflourm roots: A comprehensive study on odor/aroma and chemical properties during roasting. J. Food Biochem. 2020, 44, e13344. [Google Scholar] [CrossRef]
- Kim, D.S.; Lee, T.J.; Hong, S.J.; Cho, J.J.; Shin, E.C. Thermal coursed effect of comprehensive changes in the flavor/taste of Cynanchi wilfordii. J. Food Sci. 2019, 84, 2831–2839. [Google Scholar] [CrossRef]
- Kato, H.; Rhue, M.R.; Nishimura, T. Role of Free Amino Acids and Peptides in Food Taste in Flavor Chemistry: Trends and Developments; American Chemical Society: Washington, DC, USA, 1989; pp. 158–174. [Google Scholar]
- Lee, J.; Kim, D.S.; Cho, J.J.; Hong, S.J.; Pan, J.H.; Kim, J.K.; Shin, E.C. Perilla frutescens Britton: A comprehensive study on flavor/taste and chemical properties during the roasting process. Molecule 2019, 24, 1374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Jin, Q.; Liu, Y.; Huang, J.; Wang, X.; Mao, W.; Wang, S. Changes in volatile compounds of peanut oil during the roasting process for production of aromatic roasted peanut oil. J. Food Sci. 2011, 76, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Andersen, P.C.; Hill, K.; Gorbet, D.W.; Brodbeck, B.V. Fatty acid and amino acid profiles of selected peanut cultivars and breeding lines. J. Food Compos. Anal. 1998, 11, 100–111. [Google Scholar] [CrossRef]
- Kwak, B.M.; Kim, S.H.; Kim, K.S.; Lee, K.W.; Ahn, J.H.; Jang, C.H. Composition of vitamin A, E, B1 and B2 contents in Korean cow’s raw milk in Korea. Korean J. Food Sci. Anim. Res. 2006, 26, 245–251. [Google Scholar]
- Ei-Hazmi, M.F.; Warsy, A.S. Riboflavin status in a Saudi population: A study in Riyadh. Ann. Nutr. Metab. 1987, 31, 253–258. [Google Scholar] [CrossRef]
- Chung, J.S.; Chun, H.K.; Park, H.J. Effects of cooking method on the vitamin and mineral contents in frequently used vegetables. Korean J. Food Cook. Sci. 2016, 32, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Jackson, J.A.; Bums, M.J. Effects of cystine, niacin and taurine on cholesterol concentration in the Japanese quail with comments on bile acid metabolism. Comp. Biochem. Physio. 1974, 48, 61–68. [Google Scholar] [CrossRef]
- Jung, J.K.; Ko, S.H.; Oh, S.W.; Lim, J.Y.; Chun, T.H.; Kim, S.A.; Myoung, K.S.; Jang, C.S.; Han, Y.S. Fermentation and microbial characteristics of Korean traditional fermented milk, tarak. J. Korean Soc. Food Sci. Nutr. 2015, 44, 602–609. [Google Scholar] [CrossRef]
- Hong, S.J.; Cho, J.J.; Boo, C.G.; Yoon, M.Y.; Lee, S.M.; Shin, E.C. Comparison of physicochemical and sensory properties of bean sprout and peanut sprout extracts, subsequent to roasting. J. Korean Soc. Food Sci. Nutr. 2020, 49, 356–369. [Google Scholar] [CrossRef]
- Reineccius, G. Flavor technology. In Flavor Chemistry and Technology, 2nd ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2006; pp. 103–137. [Google Scholar]
- Gu, Y.R.; Kim, S.W.; Hong, J.H. Whitening and anti-wrinkle effects of Glehnia Radix leaf extracts according to extraction solvent. J. Korean Soc. Food Sci. Nutr. 2018, 47, 1101–1111. [Google Scholar] [CrossRef]
- Grosch, W. Instrumental and Sensory Analysis of Coffee Volatilities. In Proceedings of the 16th ASIC Colloquium (Kyoto), ASIC, Paris, France, 9–14 April 1995; pp. 147–156. [Google Scholar]
- Drake, C.L.; Jefferson, C.; Roehrs, T.; Roth, T. Stress-related sleep disturbance and polysomnographic response to caffeine. Sleep Med. 2008, 7, 567–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cha, H.S.; Park, M.S. Physiological activities of Rubus coreanus Miquel. Korean J. Food Sci. Technol. 2001, 33, 409–415. [Google Scholar]
- Kim, M.H.; Kim, S.H.; Cho, H.S.; Park, B.H. Quality characteristics of Cheonggukjang added with peanut (Arachis hypogaea L.) powder. Korean J. Food Preserv. 2016, 23, 488–494. [Google Scholar] [CrossRef] [Green Version]
- Park, N.Y. Quality characteristics and antioxidant activity of Sikhe prepared using hot water extracts of roasted coffee ground residue. Korean J. Food Sci. Technol. 2014, 46, 470–476. [Google Scholar] [CrossRef] [Green Version]
- Druaux, C.; Voilley, H.A. Effect of food composition and microstructure on volatile flavor release. Trends Food Sci. Tech. 1997, 9, 364–368. [Google Scholar] [CrossRef]
- Siegmund, B.; Murkovic, M. Changes in chemical composition of pumpkin seeds during the roasting process for production of pumpkin seed oil. Food Chem. 2004, 84, 367–374. [Google Scholar] [CrossRef]
- Min, J.S.; Kwon, H.M.; Park, S.K. Impacts of coffee creamer, dried skim milk and sugar on the volatile aroma compounds and sensory characteristics in instant coffee. J. Food Sci. Technol. 2015, 47, 137–144. [Google Scholar]
- Sereshti, H.; Samadi, S. A rapid and simple determination of caffeine in teas, coffees and eight beverages. Food Chem. 2014, 158, 8–13. [Google Scholar] [CrossRef]
- Dong, H.M.; Moon, J.Y.; Lee, S.H. Discrimination of geographical origins of raw ginseng using the electronic tongue. Korean J. Food Sci. Technol. 2017, 49, 349–354. [Google Scholar]
- Jeon, S.Y.; Kim, G.C.; Choi, S.Y.; Kim, S.B.; Kim, K.M. Analysis of electronic nose and electronic tongue and sensory characteristics of commercial seasonings. Korean J. Food Cook. Sci. 2017, 33, 538–550. [Google Scholar] [CrossRef]

| Amino Acid | Constituent Amino Acid Composition(%) | ||
| Normal Coffee | Conventional Peanut Coffee | High-Oleic Peanut Coffee | |
| Aspartic acid | 5.42 ± 0.06 a (1) | 7.51 ± 0.27 a | 8.26 ± 2.99 a |
| Threonine | N.D. (2) | 0.80 ± 0.12 a | N.D. |
| Serine | 0.80 ± 0.01 c | 2.65 ± 0.06 a | 1.37 ± 0.10 b |
| Glutamic acid | 32.82 ± 0.19 b | 33.33 ± 0.09 b | 37.28 ± 1.24 a |
| Glycine | 6.24 ± 0.01 c | 8.66 ± 0.03 a | 7.06 ± 0.23 b |
| Alanine | 3.74 ± 0.02 b | 4.03 ± 0.01 a | 3.52 ± 0.18 b |
| Cysteine | 13.63 ± 0.07 a | 9.15 ± 0.21 c | 10.59 ± 0.32 b |
| Valine | 2.56 ± 0.03 a | 2.45 ± 0.00 a | 1.79 ± 0.10 b |
| Leucine | N.D. | 1.96 ± 0.00 a | N.D. |
| Tyrosine | 11.81 ± 0.31 a | 8.42 ± 0.23 c | 9.53 ± 0.08 b |
| Phenylalanine | 16.52 ± 0.02 a | 13.06 ± 0.17 c | 14.67 ± 0.29 b |
| Arginine | N.D. | 2.14 ± 0.02 a | N.D. |
| Hydroxylysine | N.D. | 0.63 ± 0.03 a | 0.37 ± 0.26 ab |
| Unidentified amino acids | 6.45 ± 0.02 a | 5.19 ± 0.00 c | 5.67 ± 0.24 b |
| Total | 100 | 100 | 100 |
| Amino Acid | Free Amino Acid Composition(%) | ||
| Normal Coffee | Conventional Peanut Coffee | High-Oleic Peanut Coffee | |
| Serine | N.D. | 2.80 ± 0.66 a | 2.10 ± 0.41 b |
| Glycine | N.D. | 3.95 ± 0.74 a | 4.19 ± 1.46 a |
| Alanine | 5.10 ± 0.06 b | 5.69 ± 1.16 b | 10.13 ± 1.51 a |
| Valine | N.D. | 10.29 ± 1.97 a | 14.98 ± 4.59 a |
| Cysteine | N.D. | 15.93 ± 3.45 a | 8.03 ± 5.68 ab |
| Isoleucine | N.D. | 1.21 ± 0.55 a | N.D. |
| Leucine | N.D. | 3.66 ± 0.68 b | 4.51 ± 0.67 a |
| Phenylalanine | N.D. | 29.60 ± 6.35 a | 30.00 ± 4.50 a |
| Hydroxylysine | N.D. | 12.84 ± 8.16 a | N.D. |
| Unidentified amino acids | 94.90 ± 0.25 a | 14.01 ± 2.60 c | 28.42 ± 4.07 b |
| Total | 100 | 100 | 100 |
| Sample | Thiamine | Riboflavin | Niacin | Glucose | Sucrose | Caffeine |
|---|---|---|---|---|---|---|
| (mg/100 g) | (mg/100 g) | (mg/100 g) | (mg/mL) | (mg/mL) | (mg/mL) | |
| Normal coffee | 0.06 ± 0.01 c (1) | N.D. (2) | 0.63 ± 0.05 b | 1.46 ± 0.08 b | 0.07 ± 0.07 c | 16.67 ± 0.38 a |
| Conventional peanut coffee | 0.09 ± 0.01 b | N.D. | 0.82 ± 0.01 a | 1.67 ± 0.11 b | 1.12 ± 0.03 a | 15.00 ± 0.08 b |
| High-oleic peanut coffee | 0.11 ± 0.00 a | N.D. | 0.78 ± 0.02 a | 2.19 ± 0.16 a | 0.68 ± 0.15 b | 9.98 ± 0.27 c |
| Sample | DPPH-SC50 (1) | TPC (2) | pH |
|---|---|---|---|
| (mg) | (mg/mL) | ||
| Normal coffee | 61 ± 3.93 a (3) | 29.92 ± 4.17 a | 6.37 ± 0.07 b |
| Conventional peanut coffee | 55 ± 3.28 a | 35.78 ± 0.16 a | 6.59 ± 0.05 a |
| High-oleic peanut coffee | 54 ± 0.67 a | 34.31 ± 0.67 a | 6.71 ± 0.04 a |
| Volatile Compounds | RT (1) | RI (2) | NC (3) | CPC (4) | HPC (5) | I.D. (6) |
|---|---|---|---|---|---|---|
| (min) | (μg/100 g) | (μg/100 g) | (μg/100 g) | |||
| Alcohols(14) | ||||||
| 2-Furanmethanol | 10.25 | 878 | N.D. (7) | 0.009 ± 0.007 | N.D. | MS (8) |
| 3-Pyridinol | 18.11 | 1119 | N.D. | 0.005 ± 0.007 | N.D. | MS |
| Maltol | 18.54 | 1134 | N.D. | 0.004 ± 0.006 | N.D. | MS/RI |
| 4-Amino-2,6-dimethylphenol | 20.78 | 1210 | N.D. | N.D. | 0.004 ± 0.006 | MS |
| 1,2-Benzenediol | 20.90 | 1215 | N.D. | 0.003 ± 0.004 | N.D. | MS |
| 5-Endo-hydroxy-protoadamantane | 20.92 | 1215 | N.D. | N.D. | 0.002 ± 0.002 | MS |
| 4-Vinylphenol | 21.45 | 1235 | N.D. | N.D. | 0.007 ± 0.004 | MS |
| 2-Naphthalenol | 21.67 | 1243 | N.D. | N.D. | 0.002 ± 0.002 | MS |
| 4-Ethyl-2-methoxyphenol | 23.20 | 1297 | N.D. | N.D. | 0.006 ± 0.002 | MS |
| 2-Methoxy-benzeneethanol | 23.21 | 1298 | N.D. | 0.002 ± 0.003 | N.D. | MS |
| 4-Ethyl-2-methoxy-phenol | 23.22 | 1298 | 0.002 ± 0.003 | N.D. | N.D. | MS |
| 2-Methoxy-4-vinylphenol | 24.14 | 1335 | 0.014 ± 0.006 | 0.018 ± 0.001 | N.D. | MS |
| 2-Allyl-3-methoxyphenol | 25.46 | 1385 | 0.002 ± 0.002 | N.D. | N.D. | MS |
| Eugenol | 25.46 | 1385 | N.D. | N.D. | 0.005 ± 0.003 | MS/RI |
| Aldehydes(7) | ||||||
| 5-Methyl-2-furancarboxaldehyde | 13.85 | 984 | 0.001 ± 0.001 | N.D. | N.D. | MS |
| 5-Methyl-2-furfural | 13.90 | 986 | N.D. | 0.003 ± 0.002 | 0.003 ± 0.004 | MS |
| 5-(Dimethylamino)pent-2-en-4-ynal | 17.37 | 1094 | 0.003 ± 0.005 | N.D. | N.D. | MS |
| Nonanal | 18.24 | 1124 | 0.005 ± 0.007 | N.D. | N.D. | MS/RI |
| 3-Hexene-1,6-dialdehyde | 25.31 | 1379 | 0.014 ± 0.019 | N.D. | N.D. | MS |
| 4-Methyl-2,5-dimethoxybenzaldehyde | 30.23 | 1581 | N.D. | 0.002 ± 0.002 | N.D. | MS |
| 5-Methylisophthalaldehyde | 30.25 | 1582 | N.D. | 0.001 ± 0.001 | N.D. | MS |
| Hydrocarbons(8) | ||||||
| 1,2-Bis(trimethylsilyl)benzene | 9.36 | 853 | N.D. | N.D. | 0.001 ± 0.001 | MS |
| Toluene-2,4-diamine | 15.19 | 1026 | N.D. | N.D. | 0.003 ± 0.004 | MS |
| 2-Menthene | 18.19 | 1122 | N.D. | 0.002 ± 0.003 | N.D. | MS |
| 6-Aza-5,7,12,14-tetrathia pentadecene | 19.81 | 1177 | 0.008 ± 0.011 | N.D. | N.D. | MS |
| 1-(1-Propynyl)-cyclohexene | 21.45 | 1235 | N.D. | 0.002 ± 0.003 | N.D. | MS |
| β-Bourbonene | 23.11 | 1294 | 0.002 ± 0.003 | N.D. | N.D. | MS |
| 2-Methyladamantane | 23.89 | 1325 | N.D. | N.D. | 0.003 ± 0.004 | MS |
| 3,4-Dimethoxystyrene | 25.46 | 1385 | N.D. | 0.002 ± 0.002 | N.D. | MS |
| Acids and esters(8) | ||||||
| Cascarillic acid | 18.26 | 1125 | N.D. | N.D. | 0.002 ± 0.003 | MS |
| Methyl N-hydroxybenzenecarboximidoate | 12.09 | 933 | N.D. | 0.020 ± 0.029 | N.D. | MS |
| Quinic acid | 30.94 | 1612 | N.D. | 0.031 ± 0.044 | N.D. | MS |
| Isobutyric Acid | 30.96 | 1612 | N.D. | N.D. | 0.004 ± 0.006 | MS |
| 1-O-Ethyl 2-O-[2-(2-nitrophenyl)ethyl] benzene-1,2-dicarboxylate | 30.90 | 1609 | N.D. | N.D. | 0.002 ± 0.002 | MS |
| Diethyl 2-hydroxy-3-(tetrahydrofuran-2-yl)succinate | 30.98 | 1613 | N.D. | N.D. | 0.005 ± 0.008 | MS |
| 2,2,4-Trimethyl-pentan-1,3-diol diisobutyrate | 30.98 | >1700 | 0.003 ± 0.005 | N.D. | N.D. | MS |
| Phthalic acid, 5-methylhex-2-yl butyl ester | 38.89 | >1700 | N.D. | N.D. | 0.002 ± 0.002 | MS/RI |
| Heterocyclics(35) | ||||||
| N-ethyl-1,3-dithioisoindoline | 8.92 | 840 | 0.007 ± 0.010 | N.D. | N.D. | MS |
| 2-Methyl-1-vinylimidazole | 12.19 | 936 | N.D. | N.D. | 0.000 ± 0.001 | MS |
| Furfuryl acetate | 14.76 | 1099 | 0.001 ± 0.001 | 0.002 ± 0.003 | N.D. | MS |
| 3-Butynyl 4-(N-methylacetamido) benzenesulfonate | 15.16 | 1025 | 0.001 ± 0.001 | N.D. | N.D. | MS |
| Ethyl 2-(3,4-dimethylanilino)-2-oxoacetate | 16.58 | 1430 | 0.075 ± 0.106 | N.D. | N.D. | MS |
| 2-Ethyl-3,6-dimethylpyrazine | 17.53 | 1098 | N.D. | 0.007 ± 0.002 | N.D. | MS |
| 3-Ethyl-2,5-dimethyl-pyrazine | 17.545 | 1199 | 0.007 ± 0.007 | N.D. | 0.007 ± 0.001 | MS |
| Di-α-furylmethane | 17.63 | 1102 | N.D. | 0.001 ± 0.002 | N.D. | MS |
| 2,2′-Methylenebis-furan | 17.66 | 1103 | 0.002 ± 0.003 | 0.005 ± 0.007 | 0.014 ± 0.003 | MS |
| Furfuryl propinoate | 17.75 | 1106 | N.D. | 0.001 ± 0.002 | 0.003 ± 0.005 | MS |
| 4-Ethyl-2,5,6-trimethylpyrimidine | 19.84 | 1178 | N.D. | 0.005 ± 0.001 | N.D. | MS |
| 5-Methyl-2-furfurylfuran | 20.48 | 1199 | N.D. | N.D. | 0.010 ± 0.001 | MS |
| 2-(2-furanylmethyl)-5-Methylfuran | 20.50 | 1202 | 0.010 ± 0.004 | 0.004 ± 0.006 | N.D. | MS |
| N-Furfuryl pyrrole | 20.54 | 1201 | N.D. | 0.003 ± 0.003 | 0.016 ± 0.004 | MS |
| 1-(2-furanylmethyl)-1H-Pyrrole | 20.57 | 1211 | 0.014 ± 0.008 | 0.008 ± 0.011 | N.D. | MS |
| 3-Ethyl-2-formylthiophene | 20.80 | 1211 | N.D. | 0.003 ± 0.004 | N.D. | MS |
| 2,3-Dihydro-6-methylthieno [2,3c]furan | 20.80 | 1211 | 0.002 ± 0.003 | N.D. | N.D. | MS |
| 2-Dimethylamino-4,5-dimethyl-oxazole | 20.81 | 1211 | N.D. | N.D. | 0.003 ± 0.005 | MS |
| 2,3-Dihydrobenzofuran | 21.45 | 1235 | N.D. | 0.006 ± 0.009 | N.D. | MS |
| Furfuryl isovalerate | 21.58 | 1239 | N.D. | N.D. | 0.002 ± 0.003 | MS |
| Furfuryl 3-methyl butanoate | 21.58 | 1240 | 0.002 ± 0.002 | 0.001 ± 0.002 | 0.002 ± 0.002 | MS |
| 2-(2′,4′,5′-trimethylphenyl)-Propylene oxide | 23.11 | 1294 | N.D. | 0.002 ± 0.004 | N.D. | MS |
| 2,2′-(oxydimethylene)Di furan | 23.78 | 1320 | N.D. | 0.009 ± 0.003 | 0.013 ± 0.003 | MS |
| 2,2′-Difurfuryl ester | 23.80 | 1436 | 0.006 ± 0.003 | N.D. | N.D. | MS |
| 2-Methyl-6-hydroxyquinoline | 24.98 | 1367 | N.D. | N.D. | 0.003 ± 0.004 | MS |
| 5-Methyl[1,2,4]triazolo[1,5-a]pyrimidine | 26.21 | 1414 | N.D. | N.D. | 0.001 ± 0.001 | MS |
| 1-Furfuryl-2-formyl pyrrole | 26.72 | 1495 | 0.008 ± 0.003 | 0.008 ± 0.002 | 0.012 ± 0.006 | MS |
| Pyroquilon | 27.27 | 1458 | N.D. | N.D. | 0.003 ± 0.005 | MS |
| 3,4,5,6-Tetrahydro-2H-1,6-benzoxazocine | 27.49 | 1462 | N.D. | N.D. | 0.005 ± 0.001 | MS |
| 1-Furfuryl-2-acetyl pyrrole | 28.20 | 1611 | 0.002 ± 0.003 | 0.003 ± 0.004 | 0.010 ± 0.008 | MS |
| Ethyl phthalate | 30.92 | 1613 | 0.002 ± 0.000 | 0.002 ± 0.003 | N.D. | MS |
| Caffeine | 36.26 | >1700 | 0.109 ± 0.020 | 0.067 ± 0.001 | 0.089 ± 0.055 | MS |
| Butyl phthalate | 38.88 | >1700 | 0.004 ± 0.006 | N.D. | N.D. | MS |
| Dibutyl phthalate | 38.88 | >1700 | N.D. | 0.003 ± 0.005 | N.D. | |
| Phthalic acid, 2-ethylhexyl isohexyl ester | 38.88 | >1700 | 0.001 ± 0.002 | N.D. | N.D. | MS |
| Ketones(14) | ||||||
| Butyrolactone | 12.52 | 946 | N.D. | 0.002 ± 0.003 | N.D. | MS |
| 3-Chromanone | 17.69 | 1104 | 0.002 ± 0.002 | N.D. | N.D. | MS |
| 5,5,6-Trimethylbicyclo[2.2.1]heptan-2-one | 22.41 | 1270 | N.D. | N.D. | 0.002 ± 0.003 | MS |
| 3′,5′-Dihydroxyacetophenone | 23.15 | 1296 | N.D. | 0.002 ± 0.002 | N.D. | MS |
| 7,7-Dimethylbicyclo[3.3.0]octan-2-one | 23.19 | 1297 | N.D. | 0.001 ± 0.002 | N.D. | MS |
| 4′-Methoxyacetophenone | 23.90 | 1325 | N.D. | N.D. | 0.001 ± 0.002 | MS |
| 1-(5(methyl-2-furanyl)-1-Buten-3-one | 23.90 | 1325 | N.D. | 0.002 ± 0.002 | N.D. | MS |
| 2(1H)-Leoidinone | 24.99 | 1368 | N.D. | 0.001 ± 0.002 | N.D. | MS |
| 2-Methyl-4-quinazolinone | 25.19 | 1375 | N.D. | N.D. | 0.002 ± 0.002 | MS |
| 4-Methyl-6(2-methylpropenyl)-2H-pyran-2-one | 25.45 | 1385 | 0.002 ± 0.002 | N.D. | N.D. | MS |
| β-Damascenone | 26.00 | 1406 | 0.003 ± 0.005 | 0.003 ± 0.004 | 0.003 ± 0.004 | MS/RI |
| Megastigmatrienone | 30.64 | 1598 | 0.002 ± 0.003 | 0.005 ± 0.001 | 0.006 ± 0.008 | MS |
| 3,6,7,8-Tetrahydro-5-propyl-2H-azulen-1(2H)-one | 31.68 | 1644 | N.D. | 0.003 ± 0.004 | N.D. | MS |
| 2-Methylenecyclododecanone | 37.83 | >1700 | N.D. | 0.003 ± 0.004 | N.D. | MS |
| Major Compounds | RT (1) | RI (2) | Odor Intensity | Odor Description | I.D. (6) | ||
|---|---|---|---|---|---|---|---|
| (min) | NC (3) | CPC (4) | HPC (5) | ||||
| 2-Ethyl-3,6-dimethy pyrazine | 17.53 | 1098 | N.D. (7) | 1 | N.D. | Peanut | MS/GC-O (8) |
| 3-Ethyl-2,5-dimethyl pyrazine | 17.55 | 1199 | 1 | N.D. | 1 | Sweet, Peanut | MS/GC-O |
| 2,2′-Methylenebis-furan | 17.66 | 1103 | 1 | 1 | 1 | Roasted, Peanut | MS/GC-O |
| Furfuryl propinonate | 17.75 | 1106 | N.D. | 1 | 1 | Roasted | MS.GC-O |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Hong, S.J.; Cho, J.-J.; Boo, C.G.; Kim, D.-S.; Shin, E.-C. Peanut Coffee: Enhancement of Nutritional, Physicochemical, and Sensory Characteristics in Coffee Brewed with Conventional and High-Oleic Peanut Extracts. Foods 2020, 9, 1664. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9111664
Lee J, Hong SJ, Cho J-J, Boo CG, Kim D-S, Shin E-C. Peanut Coffee: Enhancement of Nutritional, Physicochemical, and Sensory Characteristics in Coffee Brewed with Conventional and High-Oleic Peanut Extracts. Foods. 2020; 9(11):1664. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9111664
Chicago/Turabian StyleLee, Jookyeong, Seong Jun Hong, Jin-Ju Cho, Chang Guk Boo, Da-Som Kim, and Eui-Cheol Shin. 2020. "Peanut Coffee: Enhancement of Nutritional, Physicochemical, and Sensory Characteristics in Coffee Brewed with Conventional and High-Oleic Peanut Extracts" Foods 9, no. 11: 1664. https://0-doi-org.brum.beds.ac.uk/10.3390/foods9111664