Protein-Based Nanostructures for Food Applications
Abstract
:1. Introduction
2. Proteins and Their Functionality
2.1. Milk Proteins
2.2. Soy Protein
2.3. Other Proteins
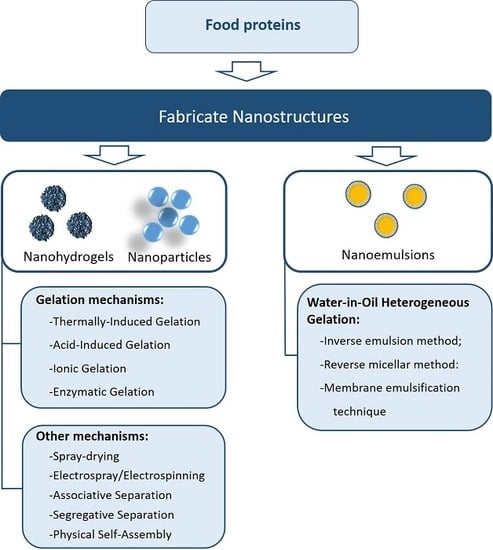
3. Methodologies to Fabricate Protein-Based Nanostructures
3.1. Gelation Mechanisms
3.1.1. Denaturation of Globular Proteins
Thermally-Induced Gelation
Acid-Induced Gelation
Ionic Gelation
Enzymatic Gelation
3.2. Other Methods and Materials to Fabricate Protein-Based Nanostructures
3.2.1. Associative Separation
3.2.2. Segregative Separation
3.2.3. Physical Self-Assembly of Interactive Polymers
3.2.4. Water-in-Oil Heterogeneous Gelation
3.2.5. Micromolding, Photolithography, and Microfluidic Preparation
3.2.6. Spray-Drying
3.2.7. Electrospray/Electrospinning
4. Protein-Based Nanostructures: A Vehicle to Transport Bioactive Compounds
5. Controlled Release
6. Conclusions
Funding
Conflicts of Interest
References
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Daniel-da-Silva, A.L.; Ferreira, L.; Gil, A.M.; Trindade, T. Synthesis and swelling behavior of temperature responsive κ-carrageenan nanogels. J. Colloid Interface Sci. 2011, 355, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Belščak-Cvitanović, A.; Komes, D.; Karlović, S.; Djaković, S.; Spoljarić, I.; Mršić, G.; Ježek, D. Improving the controlled delivery formulations of caffeine in alginate hydrogel beads combined with pectin, carrageenan, chitosan and psyllium. Food Chem. 2015, 167, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.-H.; McClements, D.J. Formation of Hydrogel Particles by Thermal Treatment of β-Lactoglobulin−Chitosan Complexes. J. Agric. Food Chem. 2007, 55, 5653–5660. [Google Scholar] [CrossRef] [PubMed]
- Abaee, A.; Mohammadian, M.; Jafari, S.M. Whey and soy protein-based hydrogels and nano-hydrogels as bioactive delivery systems. Trends Food Sci. Technol. 2017, 70, 69–81. [Google Scholar] [CrossRef]
- Morimoto, N.; Nomura, S.M.; Miyazawa, N.; Akiyoshi, K. Nanogel Engineered Designs for Polymeric Drug Delivery. In Polymeric Drug Delivery II; American Chemical Society: Washington, DC, USA, 2006; Volume 924, pp. 6–88. [Google Scholar]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Ayame, H.; Morimoto, N.; Akiyoshi, K. Self-Assembled Cationic Nanogels for Intracellular Protein Delivery. Bioconjug. Chem. 2008, 19, 882–890. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Protein-stabilized emulsions. Curr. Opin. Colloid Interface Sci. 2004, 9, 305–313. [Google Scholar] [CrossRef]
- Kellerby, S.S.; McClements, D.J.; Decker, E.A. Role of Proteins in Oil-in-Water Emulsions on the Stability of Lipid Hydroperoxides. J. Agric. Food Chem. 2006, 54, 7879–7884. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; McClements, D.J. Formation and stabilization of nanoemulsions using biosurfactants: Rhamnolipids. J. Colloid Interface Sci. 2016, 479, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, S.; Ko, S.; Xia, L.o. Use of whey proteins for encapsulation and controlled delivery applications. J. Food Eng. 2007, 83, 31–40. [Google Scholar] [CrossRef]
- Davidov-Pardo, G.; Joye, I.J.; McClements, D.J. Food-Grade Protein-Based Nanoparticles and Microparticles for Bioactive Delivery: Fabrication, Characterization, and Utilization. Adv. Protein Chem. Struct. Biol. 2015, 98, 293–325. [Google Scholar] [PubMed]
- Somchue, W.; Sermsri, W.; Shiowatana, J.; Siripinyanond, A. Encapsulation of α-tocopherol in protein-based delivery particles. Food Res. Int. 2009, 42, 909–914. [Google Scholar] [CrossRef]
- Anema, S.G.; de Kruif, C.G. Complex coacervates of lactotransferrin and β-lactoglobulin. J. Colloid Interface Sci. 2014, 430, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Augustin, M.A.; Oliver, C.M. Use of Milk Proteins for Encapsulation of Food Ingredients. Microencapsul. Food Ind. 2014, 211–226. [Google Scholar] [CrossRef]
- Chen, L. 22 Protein Micro/Nanoparticles for Controlled Nutraceutical Delivery in Functional Foods. In Woodhead Publishing Series in Food Science, Technology and Nutrition; McClements, D.J., Decker, E.A., Eds.; Woodhead Publishing: Cambridge, UK, 2009; pp. 572–600. [Google Scholar]
- Oh, J.K.; Lee, D.I.; Park, J.M. Biopolymer-based microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2009, 34, 1261–1282. [Google Scholar] [CrossRef]
- Martins, J.T.; Bourbon, A.I.; Pinheiro, A.C.; Fasolin, L.H.; Vicente, A.A. Protein-Based Structures for Food Applications: From Macro to Nanoscale. Front. Sustain. Food Syst. 2018, 2, 77. [Google Scholar] [CrossRef]
- Oliver, C.M.; Melton, L.D.; Stanley, R.A. Creating Proteins with Novel Functionality via the Maillard Reaction: A Review. Crit. Rev. Food Sci. Nutr. 2006, 46, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Verhoeckx, K.C.M.; Vissers, Y.M.; Baumert, J.L.; Faludi, R.; Feys, M.; Flanagan, S.; Herouet-Guicheney, C.; Holzhauser, T.; Shimojo, R.; van der Bolt, N.; et al. Food processing and allergenicity. Food Chem. Toxicol. 2015, 80, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Livney, Y.D. Milk proteins as vehicles for bioactives. Curr. Opin. Colloid Interface Sci. 2010, 15, 73–83. [Google Scholar] [CrossRef]
- Ramos, O.L.; Pereira, R.N.; Martins, A.; Rodrigues, R.; Fuciños, C.; Teixeira, J.A.; Pastrana, L.; Malcata, F.X.; Vicente, A.A. Design of whey protein nanostructures for incorporation and release of nutraceutical compounds in food. Crit. Rev. Food Sci. Nutr. 2017, 57, 1377–1393. [Google Scholar] [CrossRef] [PubMed]
- Creamer, L.K.; MacGibbon, A.K.H. Some recent advances in the basic chemistry of milk proteins and lipids. Int. Dairy J. 1996, 6, 539–568. [Google Scholar] [CrossRef]
- Madureira, A.R.; Pereira, C.I.; Gomes, A.M.P.; Pintado, M.E.; Malcata, F.X. Bovine whey proteins—Overview on their main biological properties. Food Res. Int. 2007, 40, 1197–1211. [Google Scholar] [CrossRef]
- Bryant, C.M.; McClements, D.J. Molecular basis of protein functionality with special consideration of cold-set gels derived from heat-denatured whey. Trends Food Sci. Technol. 1998, 9, 143–151. [Google Scholar] [CrossRef]
- O’Neill, G.J.; Jacquier, J.C.; Mukhopadhya, A.; Egan, T.; O’Sullivan, M.; Sweeney, T.; O’Riordan, E.D. In vitro and in vivo evaluation of whey protein hydrogels for oral delivery of riboflavin. J. Funct. Foods 2015, 19, 512–521. [Google Scholar]
- Wong, D.W.S.; Camirand, W.M.; Pavlath, A.E.; Parris, N.; Friedman, M. Structures and functionalities of milk proteins. Crit. Rev. Food Sci. Nutr. 1996, 36, 807–844. [Google Scholar] [CrossRef] [PubMed]
- Kontopidis, G.; Holt, C.; Sawyer, L. Invited Review: β-Lactoglobulin: Binding Properties, Structure, and Function. J. Dairy Sci. 2004, 87, 785–796. [Google Scholar] [CrossRef]
- Li, J.; Yao, P. Self-Assembly of Ibuprofen and Bovine Serum Albumin−Dextran Conjugates Leading to Effective Loading of the Drug. Langmuir 2009, 25, 6385–6391. [Google Scholar] [CrossRef] [PubMed]
- Neelima, R.S.; Rajput, Y.S.; Mann, B. Chemical and functional properties of glycomacropeptide (GMP) and its role in the detection of cheese whey adulteration in milk: A review. Dairy Sci. Technol. 2013, 93, 21–43. [Google Scholar] [CrossRef] [PubMed]
- De Medeiros, B.G.; Souza, M.; Pinheiro, A.; Bourbon, A.; Cerqueira, M.; Vicente, A.; Carneiro-da-Cunha, M. Physical Characterisation of an Alginate/Lysozyme Nano-Laminate Coating and Its Evaluation on ‘Coalho’ Cheese Shelf Life. Food Bioprocess Technol. 2014, 7, 1088–1098. [Google Scholar] [CrossRef]
- Bourbon, A.I.; Cerqueira, M.A.; Vicente, A.A. Encapsulation and controlled release of bioactive compounds in lactoferrin-glycomacropeptide nanohydrogels: Curcumin and caffeine as model compounds. J. Food Eng. 2016, 180, 110–119. [Google Scholar] [CrossRef]
- Bourbon, A.I.; Pinheiro, A.C.; Cerqueira, M.A.; Rocha, C.M.R.; Avides, M.C.; Quintas, M.A.C.; Vicente, A.A. Physico-chemical characterization of chitosan-based edible films incorporating bioactive compounds of different molecular weight. J. Food Eng. 2011, 106, 111–118. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Lee, J.H.; Ng, P.K.W. Mechanical and barrier properties of biodegradable soy protein isolate-based films coated with polylactic acid. LWT Food Sci. Technol. 2007, 40, 232–238. [Google Scholar] [CrossRef]
- Wolf, W.J. Soybean proteins. Their functional, chemical, and physical properties. J. Agric. Food Chem. 1970, 18, 969–976. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Elgohary, M.M.; Kamel, N.M. Chapter Six—Implications of Protein- and Peptide-Based Nanoparticles as Potential Vehicles for Anticancer Drugs. In Protein and Peptide Nanoparticles for Drug Delivery; Donev, R., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 98, pp. 169–221. [Google Scholar]
- Shukla, R.; Cheryan, M. Zein: The industrial protein from corn. Ind. Crops Prod. 2001, 13, 171–192. [Google Scholar] [CrossRef]
- Pascoli, M.; de Lima, R.; Fraceto, L.F. Zein Nanoparticles and Strategies to Improve Colloidal Stability: A Mini-Review. Front. Chem. 2018, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Parris, N.; Cooke, P.H.; Hicks, K.B. Encapsulation of Essential Oils in Zein Nanospherical Particles. J. Agric. Food Chem. 2005, 53, 4788–4792. [Google Scholar] [CrossRef] [PubMed]
- Nedovic, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An overview of encapsulation technologies for food applications. Procedia Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, B.; Whent, M.; Yu, L.; Wang, Q. Preparation and characterization of zein/chitosan complex for encapsulation of α-tocopherol, and its in vitro controlled release study. Colloids Surf. B Biointerfaces 2011, 85, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Teng, Z.; Wang, Q. Development of Zein Nanoparticles Coated with Carboxymethyl Chitosan for Encapsulation and Controlled Release of Vitamin D3. J. Agric. Food Chem. 2012, 60, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Bodnár, I.; Alting, A.C.; Verschueren, M. Structural effects on the permeability of whey protein films in an aqueous environment. Food Hydrocoll. 2007, 21, 889–895. [Google Scholar] [CrossRef]
- Pereira, R.N.; Rodrigues, R.M.; Altinok, E.; Ramos, Ó.L.; Xavier Malcata, F.; Maresca, P.; Ferrari, G.; Teixeira, J.A.; Vicente, A.A. Development of iron-rich whey protein hydrogels following application of ohmic heating—Effects of moderate electric fields. Food Res. Int. 2017, 99, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.N.; Rodrigues, R.M.; Ramos, Ó.L.; Malcata, F.X.; Teixeira, J.A.; Vicente, A.A. Production of Whey Protein-Based Aggregates Under Ohmic Heating. Food Bioprocess Technol. 2016, 9, 576–587. [Google Scholar] [CrossRef]
- Le Bon, C.; Nicolai, T.; Durand, D. Kinetics of Aggregation and Gelation of Globular Proteins after Heat-Induced Denaturation. Macromolecules 1999, 32, 6120–6127. [Google Scholar] [CrossRef]
- Ferry, J.D. Mechanical Properties of Substances of High Molecular Weight. IV. Rigidities of Gelatin Gels; Dependence on Concentration, Temperature and Molecular Weight1. J. Am. Chem. Soc. 1948, 70, 2244–2249. [Google Scholar] [CrossRef] [PubMed]
- Saluja, A.; Kalonia, D.S. Nature and consequences of protein–protein interactions in high protein concentration solutions. Int. J. Pharm. 2008, 358, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, G.M.; Clark, A.H.; Ross-Murphy, S.B. Heat-induced gelation of globular proteins: Part 3. Molecular studies on low pH β-lactoglobulin gels. Int. J. Biol. Macromol. 2000, 28, 41–50. [Google Scholar] [CrossRef]
- Martins, J.T.; Santos, S.F.; Bourbon, A.I.; Pinheiro, A.C.; González-Fernández, Á.; Pastrana, L.M.; Cerqueira, M.A.; Vicente, A.A. Lactoferrin-based nanoparticles as a vehicle for iron in food applications—Development and release profile. Food Res. Int. 2016, 90, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Ramos, O.L.; Pereira, R.N.; Rodrigues, R.; Teixeira, J.A.; Vicente, A.A.; Malcata, F.X. Physical effects upon whey protein aggregation for nano-coating production. Food Res. Int. 2014, 66, 344–355. [Google Scholar] [CrossRef]
- Remondetto, G.E.; Paquin, P.; Subirade, M. Cold Gelation of β-lactoglobulin in the Presence of Iron. J. Food Sci. 2002, 67, 586–595. [Google Scholar] [CrossRef]
- Lucey, J.A.; Singh, H. Formation and physical properties of acid milk gels: A review. Food Res. Int. 1997, 30, 529–542. [Google Scholar] [CrossRef]
- Andoyo, R.; Guyomarc’h, F.; Famelart, M.-H. Acid gelation of whey protein microbeads of different sizes. Dairy Sci. Technol. 2016, 96, 213–225. [Google Scholar] [CrossRef]
- Thomä-Worringer, C.; Sørensen, J.; López-Fandiño, R. Health effects and technological features of caseinomacropeptide. Int. Dairy J. 2006, 16, 1324–1333. [Google Scholar] [CrossRef]
- Graveland-Bikker, J.F.; Ipsen, R.; Otte, J.; de Kruif, C.G. Influence of Calcium on the Self-Assembly of Partially Hydrolyzed α-Lactalbumin. Langmuir 2004, 20, 6841–6846. [Google Scholar] [CrossRef] [PubMed]
- Fuciños, C.; Míguez, M.; Fuciños, P.; Pastrana, L.M.; Rúa, M.L.; Vicente, A.A. Creating functional nanostructures: Encapsulation of caffeine into α-lactalbumin nanotubes. Innov. Food Sci. Emerg. Technol. 2017, 40, 10–17. [Google Scholar] [CrossRef]
- Saricay, Y.; Wierenga, P.; de Vries, R. Nanostructure development during peroxidase catalysed cross-linking of α-lactalbumin. Food Hydrocoll. 2013, 33, 280–288. [Google Scholar] [CrossRef]
- Otte, J.; Schumacher, E.; Ipsen, R.; Ju, Z.Y.; Qvist, K.B. Protease-induced gelation of unheated and heated whey proteins: Effects of pH, temperature, and concentrations of protein, enzyme and salts. Int. Dairy J. 1999, 9, 801–812. [Google Scholar] [CrossRef]
- Munialo, C.D.; Euston, S.R.; de Jongh, H.H.J. 19—Protein gels. In Woodhead Publishing Series in Food Science, Technology and Nutrition; Yada, S.E., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 501–521. [Google Scholar]
- Dhayal, S.K.; Gruppen, H.; de Vries, R.; Wierenga, P.A. Controlled formation of protein nanoparticles by enzymatic cross-linking of α-lactalbumin with horseradish peroxidase. Food Hydrocoll. 2014, 36, 53–59. [Google Scholar] [CrossRef]
- Teng, Z.; Li, Y.; Niu, Y.; Xu, Y.; Yu, L.; Wang, Q. Cationic β-lactoglobulin nanoparticles as a bioavailability enhancer: Comparison between ethylenediamine and polyethyleneimine as cationizers. Food Chem. 2014, 159, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Dhayal, S.K.; Delahaije, R.J.B.M.; de Vries, R.J.; Gruppen, H.; Wierenga, P.A. Enzymatic cross-linking of α-lactalbumin to produce nanoparticles with increased foam stability. Soft Matter 2015, 11, 7888–7898. [Google Scholar] [CrossRef] [PubMed]
- Bourbon, A.I.; Pinheiro, A.C.; Carneiro-da-Cunha, M.G.; Pereira, R.N.; Cerqueira, M.A.; Vicente, A.A. Development and characterization of lactoferrin-GMP nanohydrogels: Evaluation of pH, ionic strength and temperature effect. Food Hydrocoll. 2015, 48, 292–300. [Google Scholar] [CrossRef]
- Jones, O.G.; McClements, D.J. Stability of Biopolymer Particles Formed by Heat Treatment of β-lactoglobulin/Beet Pectin Electrostatic Complexes. Food Biophys. 2008, 3, 191–197. [Google Scholar] [CrossRef]
- Guo, X.; Guo, X.; Meng, H.; Chen, X.; Zeng, Q.; Yu, S. Influences of different pectins on the emulsifying performance of conjugates formed between pectin and whey protein isolate. Int. J. Biol. Macromol. 2019, 123, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Diniz, R.S.; Coimbra, J.S.D.R.; Teixeira, Á.V.N.C.; da Costa, A.R.; Santos, I.J.B.; Bressan, G.C.; da Cruz Rodrigues, A.M.; da Silva, L.H.M. Production, characterization and foamability of α-lactalbumin/glycomacropeptide supramolecular structures. Food Res. Int. 2014, 64, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Yue, C.; Wusigale; Ni, Y.; Liang, L. Preparation and characterization of emulsion-filled gel beads for the encapsulation and protection of resveratrol and α-tocopherol. Food Res. Int. 2018, 108, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Arab-tehrany, E.; Jacquot, M.; Gaiani, C.; Imran, M.; Desobry, S.; Linder, M. Beneficial effects and oxidative stability of polyunsaturated fatty acids. Trends Food Sci. Technol. 2012, 25, 24–33. [Google Scholar] [CrossRef]
- Bastos, L.P.H.; de Carvalho, C.W.P.; Garcia-Rojas, E.E. Formation and characterization of the complex coacervates obtained between lactoferrin and sodium alginate. Int. J. Biol. Macromol. 2018, 120, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Yao, P.; Jiang, M.; Zhang, G. Nanogels prepared by self-assembly of oppositely charged globular proteins. Biopolymers 2006, 83, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Mohammadian, M.; Salami, M.; Emam-Djomeh, Z.; Momen, S.; Moosavi-Movahedi, A.A. Gelation of oil-in-water emulsions stabilized by heat-denatured and nanofibrillated whey proteins through ion bridging or citric acid-mediated cross-linking. Int. J. Biol. Macromol. 2018, 120, 2247–2258. [Google Scholar] [CrossRef] [PubMed]
- Diba, F.S.; Boden, A.; Thissen, H.; Bhave, M.; Kingshott, P.; Wang, P.-Y. Binary colloidal crystals (BCCs): Interactions, fabrication, and applications. Adv. Colloid Interface Sci. 2018, 261, 102–127. [Google Scholar] [CrossRef] [PubMed]
- Tokle, T.; Mcclements, D.J. Food Hydrocolloids Physicochemical properties of lactoferrin stabilized oil-in-water emulsions: Effects of pH, salt and heating. Food Hydrocoll. 2011, 25, 976–982. [Google Scholar] [CrossRef]
- Zhang, Y.; Chan, H.F.; Leong, K.W. Advanced materials and processing for drug delivery: The past and the future. Adv. Drug Deliv. Rev. 2013, 65, 104–120. [Google Scholar] [CrossRef] [PubMed]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Atkinson, P.J.; Robinson, B.H.; Howe, A.M.; Pitt, A.R. Characterisation of water-in-oil microemulsions and organo-gels based on sulphonate surfactants. Colloids Surf. A Physicochem. Eng. Asp. 1995, 94, 231–242. [Google Scholar] [CrossRef]
- Hu, M.; McClements, D.J.; Decker, E.A. Lipid Oxidation in Corn Oil-in-Water Emulsions Stabilized by Casein, Whey Protein Isolate, and Soy Protein Isolate. J. Agric. Food Chem. 2003, 51, 1696–1700. [Google Scholar] [CrossRef] [PubMed]
- Shewan, H.M.; Stokes, J.R. Review of techniques to manufacture micro-hydrogel particles for the food industry and their applications. J. Food Eng. 2013, 119, 781–792. [Google Scholar] [CrossRef]
- Ghorani, B.; Tucker, N. Fundamentals of electrospinning as a novel delivery vehicle for bioactive compounds in food nanotechnology. Food Hydrocoll. 2015, 51, 227–240. [Google Scholar] [CrossRef]
- Costa, M.A.; Ramos, M.J.; Fuciños, P.E.; Teixeira, P.; Pastrana, J.A.; Cerqueira, L. Development of Bio-Based Nanostructured Systems by Electrohydrodynamic Processes. In Nanotechnology Applications in the Food Industry; Rai, V., Bai, J., Eds.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Fernandez, A.; Torres-Giner, S.; Lagaron, J.M. Novel route to stabilization of bioactive antioxidants by encapsulation in electrospun fibers of zein prolamine. Food Hydrocoll. 2009, 23, 1427–1432. [Google Scholar] [CrossRef]
- López-Rubio, A.; Lagaron, J.M. Whey protein capsules obtained through electrospraying for the encapsulation of bioactives. Innov. Food Sci. Emerg. Technol. 2012, 13, 200–206. [Google Scholar] [CrossRef]
- Chen, L.; Remondetto, G.E.; Subirade, M. Food protein-based materials as nutraceutical delivery systems. Trends Food Sci. Technol. 2006, 17, 272–283. [Google Scholar] [CrossRef]
- Fang, B.; Zhang, M.; Tian, M.; Jiang, L.; Guo, H.Y.; Ren, F.Z. Bovine lactoferrin binds oleic acid to form an anti-tumor complex similar to HAMLET. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2014, 1841, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Sneharani, A.H.; Karakkat, J.V.; Singh, S.A.; Rao, A.G.A. Interaction of Curcumin with β-Lactoglobulin—Stability, Spectroscopic Analysis, and Molecular Modeling of the Complex. J. Agric. Food Chem. 2010, 58, 11130–11139. [Google Scholar] [CrossRef] [PubMed]
- Zimet, P.; Livney, Y.D. Beta-lactoglobulin and its nanocomplexes with pectin as vehicles for ω-3 polyunsaturated fatty acids. Food Hydrocoll. 2009, 23, 1120–1126. [Google Scholar] [CrossRef]
- Zhu, K.; Ye, T.; Peng, Z.; Liu, J.; Xu, S.; Lei, J.; Deng, H.; Li, B. Nanogels fabricated by lysozyme and sodium carboxymethyl cellulose for 5-fluorouracil controlled release. Int. J. Pharm. 2013, 441, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Maya, I.J.; McClements, D.J. Biopolymer nanoparticles as potential delivery systems for anthocyanins: Fabrication and properties. Food Res. Int. 2015, 69, 1–8. [Google Scholar] [CrossRef]
- Pérez-masiá, R.; López-nicolás, R.; Jesús, M.; Ros, G.; Lagaron, J.M. Encapsulation of folic acid in food hydrocolloids through nanospray drying and electrospraying for nutraceutical applications. Food Chem. 2015, 168, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Mascaraque, L.G.; Lagarón, J.M.; López-Rubio, A. Electrosprayed gelatin submicroparticles as edible carriers for the encapsulation of polyphenols of interest in functional foods. Food Hydrocoll. 2015, 49, 42–52. [Google Scholar] [CrossRef]
- Donato-Capel, L.; Garcia-Rodenas, C.L.; Pouteau, E.; Lehmann, U.; Srichuwong, S.; Erkner, A.; Kolodziejczyk, E.; Hughes, E.; Wooster, T.J.; Sagalowicz, L. Chapter 14—Technological Means to Modulate Food Digestion and Physiological Response. In Digestion and Health; Boland, M., Golding, M., Singh, H., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 389–422. [Google Scholar]
- Deka, S.R.; Quarta, A.; di Corato, R.; Falqui, A.; Manna, L.; Cingolani, R.; Pellegrino, T. Acidic pH-Responsive Nanogels as Smart Cargo Systems for the Simultaneous Loading and Release of Short Oligonucleotides and Magnetic Nanoparticles. Langmuir 2010, 26, 10315–10324. [Google Scholar] [CrossRef] [PubMed]
- Bourbon, A.I.; Pinheiro, A.C.; Cerqueira, M.A.; Vicente, A.A. Influence of chitosan coating on protein-based nanohydrogels properties and in vitro gastric digestibility. Food Hydrocoll. 2016, 60, 109–118. [Google Scholar] [CrossRef]
- Buonocore, G.G.; del Nobile, M.A.; Panizza, A.; Bove, S.; Battaglia, G.; Nicolais, L. Modeling the Lysozyme Release Kinetics from Antimicrobial Films Intended for Food Packaging Applications. J. Food Sci. 2003, 68, 1365–1370. [Google Scholar] [CrossRef]
- Ramos, P.E.; Cerqueira, M.A.; Cook, M.T.; Bourbon, A.I.; Khutoryanskiy, V.V.; Charalampoulos, D.; Teixeira, J.A.; Vicente, A.A. Development of an immobilization system for in situ micronutrients release. Food Res. Int. 2016, 90, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Antipov, A.A.; Sukhorukov, G.B.; Donath, E.; Möhwald, H. Sustained Release Properties of Polyelectrolyte Multilayer Capsules. J. Phys. Chem. B 2001, 105, 2281–2284. [Google Scholar] [CrossRef]
- Bourbon, A.I.; Pinheiro, A.C.; Cerqueira, M.A.; Vicente, A.A. In vitro digestion of lactoferrin-glycomacropeptide nanohydrogels incorporating bioactive compounds: Effect of a chitosan coating. Food Hydrocoll. 2018, 84, 267–275. [Google Scholar] [CrossRef]


| Proteins | Functional Properties |
|---|---|
| β-lactoglobulin | Emulsifying, foaming and gelling properties |
| Bovine serum albumin | Foaming, and emulsifying properties |
| α-Lactalbumin | Gelling properties and fat and flavor binding |
| Casein | Emulsifying, foaming and gelling properties, |
| Lactoferrin | Gelling properties |
| Gelatin | Emulsifying, gelling properties |
| Soy protein | Gelling properties and thermal stability |
| Wheat proteins | |
| Corn zein |
| System | Biopolymers | Production Techniques | References |
|---|---|---|---|
| Nano-hydrogels | Lactoferrin and Glycomacropeptide | Thermal gelation | [65] |
| Particles | β-lactoglobulin and pectin | Thermal gelation | [66] |
| Coacervates | Lactotransferrin and β-lactoglobulin | Coacervation | [15] |
| Conjugates | Weight protein isolate and pectin | Coacervation | [67] |
| Hydrogels | β-lactoglobulin and chitosan | Thermal gelation | [4] |
| Supramolecular structures | α-lactalbumin and glycomacropeptide | Self-assembly | [68] |
| Biopolymer | Bioactive Compounds | Nanoencapsulation Techniques | Application | Limitations | Reference |
|---|---|---|---|---|---|
| β-lactoglobulin | Curcumin | Complex formation | Food based nanocomplex | Environmental conditions (temperature, pH, ionic strength) | [87] |
| β-lactoglobulin and pectin | ω-3 polyunsaturated fatty acids | Electrostatic nanocomplexes | Clear acid drinks | Heat stability | [88] |
| Lysozyme and Sodium Carboxymethyl Cellulose | 5-fluorouracil | Polyelectrolyte complex coacervation | Bioactive compound carrier | Burst release of the active compound | [89] |
| β-lactoglobulin and hen egg white protein | α-tocopherol | Salt-induced gelation of the proteins | Bioactive compound carrier | n.a | [14] |
| Lactoferrin and Glycomacropeptide nanohydrogels | Curcumin and caffeine | Thermal gelation | Bioactive compound carrier | Heat stability | [33] |
| Whey protein isolate | Anthocyanin rich extracts | Coacervation | Encapsulate and protect anthocyanin | Thermal degradation | [90] |
| Whey proteinconcentrate | Folic Acid | Electrospray particles | Encapsulation of bioactive compounds | n.a | [91] |
| Gelatin | Polyphenols | Electrospray particles | n.a | [92] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourbon, A.I.; Pereira, R.N.; Pastrana, L.M.; Vicente, A.A.; Cerqueira, M.A. Protein-Based Nanostructures for Food Applications. Gels 2019, 5, 9. https://0-doi-org.brum.beds.ac.uk/10.3390/gels5010009
Bourbon AI, Pereira RN, Pastrana LM, Vicente AA, Cerqueira MA. Protein-Based Nanostructures for Food Applications. Gels. 2019; 5(1):9. https://0-doi-org.brum.beds.ac.uk/10.3390/gels5010009
Chicago/Turabian StyleBourbon, Ana I., Ricardo N. Pereira, Lorenzo M. Pastrana, António A. Vicente, and Miguel A. Cerqueira. 2019. "Protein-Based Nanostructures for Food Applications" Gels 5, no. 1: 9. https://0-doi-org.brum.beds.ac.uk/10.3390/gels5010009