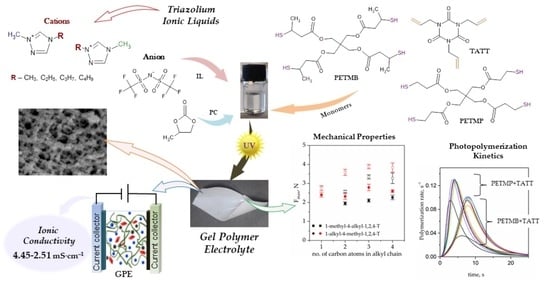
Gel Polymer Electrolytes with Mixture of Triazolium Ionic Liquids and Propylene Carbonate
Abstract
:1. Introduction
2. Materials
2.1. Reagents for Ionic Liquid Synthesis
2.2. Other Reagents
3. Methodology
3.1. Synthesis of 1,4-Dialkyl-triazolium Ionic Liquid
3.2. Solvatochromic Parameters
3.3. Isothermal Differential Scanning Photocalorimetry
3.4. Infrared Spectroscopy
3.5. Gel Polymer Electrolyte Synthesis
3.6. Puncture Resistance
3.7. Differential Scanning Calorimetry
3.8. Electrochemical Impedance Spectroscopy
3.9. Electrochemical Capacitor Investigation
3.9.1. Electrodes Preparation
3.9.2. Electrochemical Investigation
3.10. Scanning Electron Microscope
4. Results and Discussion
4.1. Characterization of Components and Compositions
4.2. Ionogel Synthesis
4.3. Photopolymerization Kinetics
4.4. Ionic Conductivity
4.5. Mechanical Properties
4.6. Investigation of Electrochemical Capacitor
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, E.; Han, J.; Ryu, S.; Choi, Y.; Yoo, J. Ionic Liquid Electrolytes for Electrochemical Energy Storage Devices. Materials 2021, 14, 4000. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, J.-G.; Xu, W. Advancing Lithium Metal Batteries. Joule 2018, 2, 833–845. [Google Scholar] [CrossRef] [Green Version]
- Tikekar, M.D.; Choudhury, S.; Tu, Z.; Archer, L.A. Design principles for electrolytes and interfaces for stable lithium-metal batteries. Nat. Energy 2016, 1, 1–7. [Google Scholar] [CrossRef]
- Larcher, D.; Tarascon, J.-M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Yarmolenko, O.V.; Yudina, A.V.; Khatmullina, K.G. Nanocomposite Polymer Electrolytes for the Lithium Power Sources (a Review). Russ. J. Electrochem. 2018, 54, 325–343. [Google Scholar] [CrossRef]
- Hoang Huy, V.P.; So, S.; Hur, J. Inorganic Fillers in Composite Gel Polymer Electrolytes for High-Performance Lithium and Non-Lithium Polymer Batteries. Nanomaterials 2021, 11, 614. [Google Scholar] [CrossRef]
- Nurul ‘Afini Mohd Johari, S. A Review: Ionic Conductivity of Solid Polymer Electrolyte Based Polyethylene Oxide. Int. J. Electrochem. Sci. 2021, 16, 2. [Google Scholar] [CrossRef]
- Siqueira, L.J.A.; Ribeiro, M.C.C. Molecular dynamics simulation of the polymer electrolyte poly(ethylene oxide)/LiClO(4). II. Dynamical properties. J. Chem. Phys. 2006, 125, 214903. [Google Scholar] [CrossRef]
- Sun, B.; Mindemark, J.; Edström, K.; Brandell, D. Polycarbonate-based solid polymer electrolytes for Li-ion batteries. Solid State Ion. 2014, 262, 738–742. [Google Scholar] [CrossRef]
- Fonseca, C.; Neves, S. Characterization of polymer electrolytes based on poly(dimethyl siloxane-co-ethylene oxide). J. Power Sources 2002, 104, 85–89. [Google Scholar] [CrossRef]
- Cho, Y.-G.; Hwang, C.; Cheong, D.S.; Kim, Y.-S.; Song, H.-K. Gel/Solid Polymer Electrolytes Characterized by In Situ Gelation or Polymerization for Electrochemical Energy Systems. Adv. Mater. 2019, 31, e1804909. [Google Scholar] [CrossRef]
- Agrawal, R.C.; Pandey, G.P. Solid polymer electrolytes: Materials designing and all-solid-state battery applications: An overview. J. Phys. D Appl. Phys. 2008, 41, 223001. [Google Scholar] [CrossRef]
- Daily, L.A.; Miller, K.M. Correlating structure with thermal properties for a series of 1-alkyl-4-methyl-1,2,4-triazolium ionic liquids. J. Org. Chem. 2013, 78, 4196–4201. [Google Scholar] [CrossRef]
- Elia, G.A.; Ulissi, U.; Jeong, S.; Passerini, S.; Hassoun, J. Exceptional long-life performance of lithium-ion batteries using ionic liquid-based electrolytes. Energy Environ. Sci. 2016, 9, 3210–3220. [Google Scholar] [CrossRef] [Green Version]
- Quartarone, E.; Mustarelli, P. Review—Emerging Trends in the Design of Electrolytes for Lithium and Post-Lithium Batteries. J. Electrochem. Soc. 2020, 167, 50508. [Google Scholar] [CrossRef]
- Zabielska-Matejuk, J.; Stangierska, A.; Kot, M. New Ammonium- and 1,2,4-Triazolium-Based Ionic Liquids for Wood Preservation. J. Wood Chem. Technol. 2015, 35, 178–192. [Google Scholar] [CrossRef]
- Cheng, W.; Chen, X.; Sun, J.; Wang, J.; Zhang, S. SBA-15 supported triazolium-based ionic liquids as highly efficient and recyclable catalysts for fixation of CO2 with epoxides. Catal. Today 2013, 200, 117–124. [Google Scholar] [CrossRef]
- Rogers, R.D.; Seddon, K.R. Ionic Liquids III A: Fundamentals, Progress, Challenges, and Opportunities; American Chemical Society: Washington, WA, USA, 2005; ISBN 9780841238930. [Google Scholar]
- Ly Nguyen, T.K.; Obadia, M.M.; Serghei, A.; Livi, S.; Duchet-Rumeau, J.; Drockenmuller, E. 1,2,3-Triazolium-Based Epoxy-Amine Networks: Ion-Conducting Polymer Electrolytes. Macromol. Rapid Commun. 2016, 37, 1168–1174. [Google Scholar] [CrossRef]
- De La Hoz, A.T.; Brauer, U.G.; Miller, K.M. Physicochemical and thermal properties for a series of 1-alkyl-4-methyl-1,2,4-triazolium bis(trifluoromethylsulfonyl)imide ionic liquids. J. Phys. Chem. B 2014, 118, 9944–9951. [Google Scholar] [CrossRef]
- Lewandowska, A.; Gajewski, P.; Szcześniak, K.; Fojud, Z.; Robakowska, M.; Skrzypczak, A.; Voelkel, A.; Marcinkowska, A. Thiol-ene ionogels based on polymerizable imidazolium ionic liquid. Polym. Chem. 2022, 13, 3154–3170. [Google Scholar] [CrossRef]
- Lewandowska, A.; Gajewski, P.; Szcześniak, K.; Sadej, M.; Patelski, P.; Marcinkowska, A. Modification of Thiol-Ene Ionogels with Octakis(methacryloxypropyl) Silsesquioxane. Polymers 2021, 13, 385. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewska, E. Photoinitiated polymerization in ionic liquids and its application. Polym. Int. 2017, 66, 366–381. [Google Scholar] [CrossRef]
- Yu, B.; Wang, X.; Xing, W.; Yang, H.; Wang, X.; Song, L.; Hu, Y.; Lo, S. Enhanced thermal and mechanical properties of functionalized graphene/thiol-ene systems by photopolymerization technology. Chem. Eng. J. 2013, 228, 318–326. [Google Scholar] [CrossRef] [Green Version]
- Mirzaei, Y.R.; Twamley, B.; Shreeve, J.M. Syntheses of 1-alkyl-1,2,4-triazoles and the formation of quaternary 1-alkyl-4-polyfluoroalkyl-1,2,4-triazolium salts leading to ionic liquids. J. Org. Chem. 2002, 67, 9340–9345. [Google Scholar] [CrossRef]
- Lewandowska, A.; Gajewski, P.; Szcześniak, K.; Marcinkowska, A. The Influence of Monomer Structure on the Properties of Ionogels Obtained by Thiol-Ene Photopolymerization. Gels 2021, 7, 214. [Google Scholar] [CrossRef]
- Taft, R.W.; Kamlet, M.J. The solvatochromic comparison method. 2. The.alpha.-scale of solvent hydrogen-bond donor (HBD) acidities. J. Am. Chem. Soc. 1976, 98, 2886–2894. [Google Scholar] [CrossRef]
- Mortimer, J. Kamlet; Jose Luis Abboud; and R. W. Taft. The solvatochromic comparison method. 6. The.pi.* scale of solvent polarities. J. Am. Chem. Soc. 1977, 99, 6027–6038. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Taft, R.W. The solvatochromic comparison method. I. The.beta.-scale of solvent hydrogen-bond acceptor (HBA) basicities. J. Am. Chem. Soc. 1976, 98, 377–383. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Abboud, J.L.M.; Abraham, M.H.; Taft, R.W. Linear solvation energy relationships. 23. A comprehensive collection of the solvatochromic parameters,.pi.*,.alpha., and.beta., and some methods for simplifying the generalized solvatochromic equation. J. Org. Chem. 1983, 48, 2877–2887. [Google Scholar] [CrossRef]
- Roper, T.M.; Guymon, C.A.; Jönsson, E.S.; Hoyle, C.E. Influence of the alkene structure on the mechanism and kinetics of thiol-alkene photopolymerizations with real-time infrared spectroscopy. J. Polym. Sci. A Polym. Chem. 2004, 42, 6283–6298. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, H.; Hoyle, C.E. The effect of thiol and ene structures on thiol–ene networks: Photopolymerization, physical, mechanical and optical properties. Polymer 2009, 50, 2237–2245. [Google Scholar] [CrossRef]
- Fouassier, J.P.; Rabek, J.F. Radiation Curing in Polymer Science and Technology; Elsevier: London, UK, 1993; ISBN 1851669299. [Google Scholar]
- Brauer, U.G.; de La Hoz, A.T.; Miller, K.M. The effect of counteranion on the physicochemical and thermal properties of 4-methyl-1-propyl-1,2,4-triazolium ionic liquids. J. Mol. Liq. 2015, 210, 286–292. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Gordon, M.S.; Boatz, J.A. Triazolium-based energetic ionic liquids. J. Phys. Chem. A 2005, 109, 7285–7295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zgrzeba, A.; Andrzejewska, E.; Marcinkowska, A. Ionic liquid-containing ionogels by thiol–ene photopolymerization. Kinetics and solvent effect. RSC Adv. 2015, 5, 100354–100361. [Google Scholar] [CrossRef]












| Name | Acronym | Yield, % |
|---|---|---|
| 1,4-dimethyl-1,2,4-triazolium bis(trifluoromethylsulfonyl)imide | MMT | 91.2 |
| 4-ethyl-1-methyl-1,2,4-triazolium bis(trifluoromethylsulfonyl)imide | MET | 92.7 |
| 1-methyl-4-propyl-1,2,4-triazolium bis(trifluoromethylsulfonyl)imide | MPT | 93.5 |
| 4-butyl-1-methyl-1,2,4-triazolium bis(trifluoromethylsulfonyl)imide | MBT | 94.0 |
| 1-ethyl-4-methyl-1,2,4-triazolium bis(trifluoromethylsulfonyl)imide | EMT | 92.1 |
| 4-methyl-1-propyl-1,2,4-triazolium bis(trifluoromethylsulfonyl)imide | PMT | 94.1 |
| 1-butyl-4-methyl-1,2,4-triazolium bis(trifluoromethylsulfonyl)imide | BMT | 86.4 |
| Sample | π* | α | β |
|---|---|---|---|
| TATT | 0.63 | 0.25 | 0.51 |
| PETMP | 0.93 | 0.49 | 0.32 |
| PETMB | 0.79 | 0.58 | 0.40 |
| MMT+PC | 0.98 | 0.78 | 0.31 |
| MET+PC | 0.98 | 0.76 | 0.31 |
| EMT+PC | 0.98 | 0.77 | 0.31 |
| MPT+PC | 0.98 | 0.74 | 0.31 |
| PMT+PC | 0.98 | 0.75 | 0.31 |
| MBT+PC | 0.98 | 0.73 | 0.31 |
| BMT+PC | 0.98 | 0.74 | 0.31 |
| Polymer | PETMP+TATT | PETMB+TATT | ||
|---|---|---|---|---|
| IL Cation | Photo | SEM | Photo | SEM |
| MMT |  +++  + |  |  +++  + |  |
| MET |  +++  ++ |  |  +++  + |  |
| MPT |  +++  ++ |  |  ++  + |  |
| MBT |  +++  + |  |  ++  + |  |
| EMT |  +++  +++ |  |  +++  + |  |
| PMT |  ++  + |  |  +  − |  |
| BMT |  ++  + |  |  +  − |  |
| Polymer Matrix | IL in PC | Particle Size, nm | Tg, °C |
|---|---|---|---|
| TATT+PETMP | - | - | 36.6 |
| MMT | 176 ± 37 | 10.3 | |
| EMT | 189 ± 44 | 7.6 | |
| MET | 160 ± 20 | 10.0 | |
| PMT | 152 ± 24 | 9.6 | |
| MPT | 155 ± 26 | 8.6 | |
| BMT | 146 ± 22 | 6.6 | |
| MBT | 122 ± 22 | 8.3 | |
| TATT+PETMB | - | - | 34.1 |
| MMT | 152 ± 25 | 18.6 | |
| EMT | 91 ± 15 | 17.6 | |
| MET | 123 ± 19 | 19.3 | |
| PMT | 99 ± 17 | 19.0 | |
| MPT | 98 ± 19 | 21.32 | |
| BMT | 64 ± 10 | 18.00 | |
| MBT | 58 ± 8 | 19.96 |
| IL in PC | Conductivity, mS·cm−1 | |
|---|---|---|
| TATT+PETMP | TATT+PETMB | |
| MMT | 4.43 ± 0.08 | 4.02 ± 0.11 |
| MET | 4.72 ± 0.15 | 4.45 ± 0.09 |
| MPT | 4.23 ± 0.13 | 3.14 ± 0.014 |
| MBT | 3.74 ± 0.09 | 2.9 ± 0.11 |
| EMT | 4.27 ± 0.06 | 3.95 ± 0.12 |
| PMT | 3.60 ± 0.11 | 2.68 ± 0.13 |
| BMT | 3.4 ± 0.07 | 2.51 ± 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewandowska, A.; Gajewski, P.; Szcześniak, K.; Marcinkowska, A. Gel Polymer Electrolytes with Mixture of Triazolium Ionic Liquids and Propylene Carbonate. Gels 2022, 8, 370. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8060370
Lewandowska A, Gajewski P, Szcześniak K, Marcinkowska A. Gel Polymer Electrolytes with Mixture of Triazolium Ionic Liquids and Propylene Carbonate. Gels. 2022; 8(6):370. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8060370
Chicago/Turabian StyleLewandowska, Aneta, Piotr Gajewski, Katarzyna Szcześniak, and Agnieszka Marcinkowska. 2022. "Gel Polymer Electrolytes with Mixture of Triazolium Ionic Liquids and Propylene Carbonate" Gels 8, no. 6: 370. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8060370