A Facile Method for Fabricating a Monolithic Mullite Fiber-Reinforced Alumina Aerogel with Excellent Mechanical and Thermal Properties
Abstract
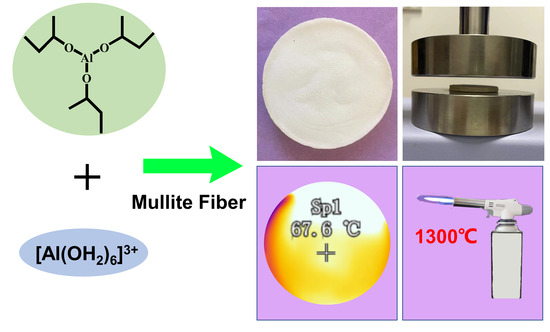
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation
4.3. Characterization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Almeida, C.M.R.; Ghica, M.E.; Durães, L. An overview on alumina-silica-based aerogels. Adv. Colloid Interface Sci. 2020, 282, 102189. [Google Scholar] [CrossRef] [PubMed]
- Du, A.; Zhou, B.; Zhang, Z.; Shen, J. A Special Material or a New State of Matter: A Review and Reconsideration of the Aerogel. Materials 2013, 6, 941–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zu, G.; Shen, J.; Wang, W.; Zou, L.; Lian, Y.; Zhang, Z.; Liu, B.; Zhang, F. Robust, Highly Thermally Stable, Core–Shell Nanostructured Metal Oxide Aerogels as High-Temperature Thermal Superinsulators, Adsorbents, and Catalysts. Chem. Mater. 2014, 26, 5761–5772. [Google Scholar] [CrossRef]
- Pierre, A.C.; Pajonk, G.M. Chemistry of Aerogels and Their Applications. Chem. Rev. 2002, 102, 4243–4266. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, X.; Shen, J. Metal oxide aerogels for high-temperature applications. J. Sol-Gel Sci. Technol. 2022; online published. [Google Scholar] [CrossRef]
- Randall, J.P.; Meador, M.A.B.; Jana, S.C. Tailoring Mechanical Properties of Aerogels for Aerospace Applications. ACS Appl. Mater. Inter. 2011, 3, 613–626. [Google Scholar] [CrossRef]
- Zu, G.; Shen, J.; Zou, L.; Wang, W.; Lian, Y.; Zhang, Z.; Du, A. Nanoengineering Super Heat-Resistant, Strong Alumina Aerogels. Chem. Mater. 2013, 25, 4757–4764. [Google Scholar] [CrossRef]
- Gao, M.; Liu, B.; Zhao, P.; Yi, X.; Shen, X.; Xu, Y. Mechanical strengths and thermal properties of titania-doped alumina aerogels and the application as high-temperature thermal insulator. J. Sol-Gel Sci. Technol. 2019, 91, 514–522. [Google Scholar] [CrossRef]
- Wang, X.; Tian, Y.; Yu, C.; Liu, L.; Zhang, Z.; Wu, Y.; Shen, J. Organic/inorganic double-precursor cross-linked alumina aerogel with high specific surface area and high-temperature resistance. Ceram. Int. 2022, 48, 17261–17269. [Google Scholar] [CrossRef]
- Zu, G.; Shen, J.; Wei, X.; Ni, X.; Zhang, Z.; Wang, J.; Liu, G. Preparation and characterization of monolithic alumina aerogels. J. Non-Cryst. Solids 2011, 357, 2903–2906. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Z.; Zu, G.; Shen, J.; Zou, L.; Lian, Y.; Liu, B.; Zhang, F. Trimethylethoxysilane-modified super heat-resistant alumina aerogels for high-temperature thermal insulation and adsorption applications. RSC Adv. 2014, 4, 54864–54871. [Google Scholar] [CrossRef]
- Baumann, T.F.; Gash, A.E.; Chinn, S.C.; Sawvel, A.M.; Maxwell, R.S.; Satcher, J.H. Synthesis of High-Surface-Area Alumina Aerogels without the Use of Alkoxide Precursors. Chem. Mater. 2005, 17, 395–401. [Google Scholar] [CrossRef] [Green Version]
- Gan, L.; Xu, Z.; Feng, Y.; Chen, L. Synthesis of Alumina Aerogels by Ambient Drying Method and Control of Their Structures. J. Porous Mat. 2005, 12, 317–321. [Google Scholar] [CrossRef]
- Li, C.; Gong, Z.; Ding, L.; Guo, D.; Hu, W. Synthesis of low shrinkage monolith alumina aerogels by surface modification and ambient pressure drying. Micro Nano Lett. 2018, 13, 1240–1244. [Google Scholar] [CrossRef]
- Poco, J.F.; Satcher, J.H.; Hrubesh, L.W. Synthesis of high porosity, monolithic alumina aerogels. J. Non-Cryst. Solids 2001, 285, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Jerzy, W.; Marek, S.; Mieczys, A.S.; Barbara, P. Synthesis and properties of alumina aerogels. React. Kinet. Catal. Lett. 1999, 66, 71–77. [Google Scholar]
- Zou, W.; Wang, X.; Wu, Y.; Zu, G.; Zou, L.; Zhang, R.; Yao, X.; Shen, J. Highly thermally stable alumina-based aerogels modified by partially hydrolyzed aluminum tri-sec-butoxide. J. Sol-Gel Sci. Technol. 2017, 84, 507–514. [Google Scholar] [CrossRef]
- Nguyen, T.; Tang, D.; Acierno, F.D.; Michal, C.A.; Maclachlan, M.J. Biotemplated Lightweight γ-Alumina Aerogels. Chem. Mater. 2018, 30, 1602–1609. [Google Scholar] [CrossRef]
- Peng, F.; Jiang, Y.; Feng, J.; Cai, H.; Feng, J.; Li, L. Research Progress on Alumina Aerogel Composites for High-temperature Thermal Insulation. J. Inorg. Mater. 2021, 36, 673–684. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, R.; Zhao, C. Ultra-small sepiolite fiber toughened alumina aerogel with enhanced thermal stability and machinability. J. Porous Mater. 2020, 27, 1535–1546. [Google Scholar] [CrossRef]
- Ya, Z.; Gaofeng, S.; Xiaodong, W.; Yong, K.; Xue, W.; Sheng, C.; Xiaodong, S. Robust monolithic polymer(resorcinol-formaldehyde) reinforced alumina aerogel composites with mutually interpenetrating networks. RSC Adv. 2019, 9, 22942–22949. [Google Scholar]
- Xu, L.; Jiang, Y.; Feng, J.; Feng, J.; Yue, C. Infrared-opacified Al2O3–SiO2 aerogel composites reinforced by SiC-coated mullite fibers for thermal insulations. Ceram. Int. 2015, 41, 437–442. [Google Scholar] [CrossRef]
- Jin, X.; Xu, J.; Pan, Y.; Wang, H.; Ma, B.; Liu, F.; Yan, X.; Wu, C.; Huang, H.; Cheng, H.; et al. Lightweight and multiscale needle quartz fiber felt reinforced siliconoxycarbide modified phenolic aerogel nanocomposite with enhanced mechanical, insulative and flame-resistant properties. Compos. Sci. Technol. 2022, 217, 109100. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, T.; Yi, Z.; Yan, L.; Liu, S.; Yao, X.; Guo, A.; Liu, J.; Hou, F. Multiscale mullite fiber/whisker reinforced silica aerogel nanocomposites with enhanced compressive strength and thermal insulation performance. Ceram. Int. 2020, 46, 28561–28568. [Google Scholar] [CrossRef]
- Yu, H.; Tong, Z.; Zhang, B.; Chen, Z.; Li, X.; Su, D.; Ji, H. Thermal radiation shielded, high strength, fire resistant fiber/nanorod/aerogel composites fabricated by in-situ growth of TiO2 nanorods for thermal insulation. Chem. Eng. J. 2021, 418, 129342. [Google Scholar] [CrossRef]
- Wu, L.H.Y.W. Preparation and Characterization of Monolithic Silica Fiber/Al2O3 Aerogel Composites by Non-Supercritical Drying. Editor. Off. Acta Phys.-Chim. Sin. 2010, 26, 1717–1721. [Google Scholar]
- Peng, F.; Jiang, Y.; Feng, J.; Cai, H.; Feng, J.; Li, L. Thermally insulating, fiber-reinforced alumina-silica aerogel composites with ultra-low shrinkage up to 1500 °C. Chem. Eng. J. 2021, 411, 128402. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, R.; Fang, D. An ultralight silica-modified ZrO2–SiO2 aerogel composite with ultra-low thermal conductivity and enhanced mechanical strength. Scr. Mater. 2018, 143, 113–116. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, R.; Fang, D. Novel whisker-reinforced Al2O3–SiO2 aerogel composites with ultra-low thermal conductivity. Ceram. Int. 2017, 43, 9547–9551. [Google Scholar] [CrossRef]
- Yu, H.; Jiang, Y.; Lu, Y.; Li, X.; Zhao, H.; Ji, Y.; Wang, M. Quartz fiber reinforced Al2O3-SiO2 aerogel composite with highly thermal stability by ambient pressure drying. J. Non-Cryst. Solids 2019, 505, 79–86. [Google Scholar] [CrossRef]
- Jaxel, J.; Markevicius, G.; Rigacci, A.; Budtova, T. Thermal superinsulating silica aerogels reinforced with short man-made cellulose fibers. Compos. Part. A Appl. Sci. Manuf. 2017, 103, 113–121. [Google Scholar] [CrossRef]
- Xue, D.; Zhong, C.; Anran, G.; Jiachen, L.; Xuan, W.; Cong, C. Mechanical and interfacial behavior of single mullite fiber and mullite fiber-based porous ceramics. Ceram. Int. 2018, 44, 14446–14456. [Google Scholar]
- Chawla, K.K.; Xu, Z.R.; Ha, J.S. Processing, structure, and properties of mullite fiber/mullite matrix composites. J. Eur. Ceram. Soc. 1996, 16, 293–299. [Google Scholar] [CrossRef]
- Zeng, S.Q.; Hunt, A.; Greif, R. Theoretical modeling of carbon content to minimize heat transfer in silica aerogel. J. Non-Cryst. Solids 1995, 186, 271–277. [Google Scholar] [CrossRef]
- Zhang, H.; He, X.; He, F. Microstructural characterization and properties of ambient-dried SiO2 matrix aerogel doped with opacified TiO2 powder. J. Alloy. Compd. 2009, 469, 366–369. [Google Scholar] [CrossRef]
- Yang, J.; Wu, H.; Huang, G.; Liang, Y.; Liao, Y. Modeling and coupling effect evaluation of thermal conductivity of ternary opacifier/fiber/aerogel composites for super-thermal insulation. Mater. Des. 2017, 133, 224–236. [Google Scholar] [CrossRef]
- Zou, W.; Wang, X.; Wu, Y.; Zou, L.; Zu, G.; Chen, D.; Shen, J. Opacifier embedded and fiber reinforced alumina-based aerogel composites for ultra-high temperature thermal insulation. Ceram. Int. 2019, 45, 644–650. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Provisional). Pure Appl. Chem. 2013, 54, 45–56. [Google Scholar] [CrossRef]
- Rodney, T.; Douglas, A.H. Crystal chemistry of boehmite. GeoScienceWorld 1980, 28, 373–380. [Google Scholar]
- Gary, G.C.; Charles, E.C.; Douglas, A.H.; Rodney, T.T. The crystal structure of boehmite. GeoScienceWorld 1979, 27, 495–501. [Google Scholar]
- Ren, H.; Wu, D.; Li, J.; Wu, W. Thermal insulation characteristics of a lightweight, porous nanomaterial in high-temperature environments. Mater. Des. 2018, 140, 376–386. [Google Scholar] [CrossRef]








| Sample | Specific Surface Area (m2/g) | |
|---|---|---|
| As Prepared | 1300 °C | |
| AM0 | 423 | 96 |
| AM1 | 407 | 117 |
| AM2 | 374 | 137 |
| AM3 | 362 | 145 |
| AM4 | 342 | 112 |
| AM5 | 261 | 99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Wang, X.; Zhang, Z.; Shi, Y.; Zhao, Y.; Shen, S.; Yao, X.; Shen, J. A Facile Method for Fabricating a Monolithic Mullite Fiber-Reinforced Alumina Aerogel with Excellent Mechanical and Thermal Properties. Gels 2022, 8, 380. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8060380
Liu L, Wang X, Zhang Z, Shi Y, Zhao Y, Shen S, Yao X, Shen J. A Facile Method for Fabricating a Monolithic Mullite Fiber-Reinforced Alumina Aerogel with Excellent Mechanical and Thermal Properties. Gels. 2022; 8(6):380. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8060380
Chicago/Turabian StyleLiu, Lin, Xiaodong Wang, Ze Zhang, Yixin Shi, Yicheng Zhao, Shiqi Shen, Xiandong Yao, and Jun Shen. 2022. "A Facile Method for Fabricating a Monolithic Mullite Fiber-Reinforced Alumina Aerogel with Excellent Mechanical and Thermal Properties" Gels 8, no. 6: 380. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8060380