Bis-Pyridine-Based Organogel with AIE Effect and Sensing Performance towards Hg2+
Abstract
:1. Introduction
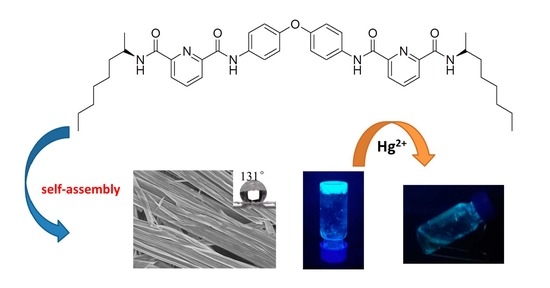
2. Results and Discussion
3. Conclusions
4. Experimental Section
4.1. Reagents and Solvents
4.2. Techniques and Instrumentations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dang, L.L.; Li, T.T.; Zhang, T.T.; Zhao, Y.; Chen, T.; Gao, X.; Ma, L.-F.; Jin, G.-X. Highly selective synthesis and near-infrared photothermal conversion of metalla-Borromean ring and [2]catenane assemblies. Chem. Sci. 2022, 13, 5130–5140. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, J.-G.; Ma, L.-L.; Li, M.; An, Y.-Y.; Han, Y.-F. Strategies for the construction of supramolecular assemblies from poly-NHC ligand precursors. Sci. China Chem. 2021, 64, 701–718. [Google Scholar] [CrossRef]
- Zhang, Y.-W.; Bai, S.; Wang, Y.-Y.; Han, Y.-F. A strategy for the construction of triply interlocked organometallic cages by rational design of poly-NHC precursors. J. Am. Chem. Soc. 2020, 142, 13614–13621. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-X.; Jiang, X.-F.; Ma, Y.-J.; Liu, X.-R.; Ge, B.-D.; Wang, A.-N.; Wei, Q.; Wang, G.-M. Optically actuating ultra-stable radicals in a large π-conjugated ligand constructed photochromic complex. Sci. China Chem. 2021, 64, 432–438. [Google Scholar] [CrossRef]
- Liu, S.-J.; Han, S.-D.; Zhao, J.-P.; Xu, J.; Bu, X.-H. In-situ synthesis of molecular magnetorefrigerant materials. Coord. Chem. Rev. 2019, 394, 39–52. [Google Scholar] [CrossRef]
- Wu, Y.P.; Tian, J.W.; Liu, S.; Li, B.; Zhao, J.; Ma, L.F.; Li, D.S.; Lan, Y.Q.; Bu, X. Bi-microporous metal-organic-frameworks with cubane [M4(OH)4] (M = Ni, Co) clusters and pore space partition for electrocatalytic methanol oxidation reaction. Angew. Chem. Int. Ed. 2019, 58, 12185–12189. [Google Scholar] [CrossRef]
- Xu, X.; Li, H.; Xie, S.; Mei, L.; Meng, R.; Chen, L.; Zhao, J. Double-oxalate−bridging tetralanthanide containing divacant Lindqvist isopolytungstates with energy transfer mechanism and luminous color adjustablility through Eu3+/Tb3+ cooping. Inorg. Chem. 2020, 59, 648–660. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, X.; Wu, Y.; Li, D.-S.; Zhang, Q. Surfactants as promising media in the field of metal-organic frameworks. Coord. Chem. Rev. 2019, 391, 30–43. [Google Scholar] [CrossRef]
- Terech, P.; Weiss, R.G. Editors Molecular Gels: Materials with Self-Assembled Fibrillar Networks; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Guenet, J.M. Organogels: Thermodynamics, Structure, Solvent Role and Properties; Springer International Publishing: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Weiss, R.G. (Ed.) Molecular Gels, Structure and Dynamics. Monograph in Supramolecular Chemistry; Royal Society of Chemistry: London, UK, 2018. [Google Scholar]
- Wu, F.; Maier, J.; Yu, Y. Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries. Chem. Soc. Rev. 2020, 49, 1569–1614. [Google Scholar] [CrossRef]
- Ji, C.; Zeng, J.; Qin, S.; Chen, M.; Wu, L. Angle-independent responsive organogel retroreflective structural color film for colorimetric sensing of humidity and organic vapors. Chin. Chem. Lett. 2021, 32, 3584–3590. [Google Scholar] [CrossRef]
- Panja, S.; Adams, D.J. Stimuli responsive dynamic transformations in supramolecular gels. Chem. Soc. Rev. 2021, 50, 5165–5200. [Google Scholar] [CrossRef] [PubMed]
- Xian, C.; Yuan, Q.; Bao, Z.; Liu, G.; Wu, J. Progress on intelligent hydrogels based on RAFT polymerization: Design strategy, fabrication and the applications for controlled drug delivery. Chin. Chem. Lett. 2020, 31, 19–27. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Y.; Gao, H.; Chang, Q.; Cheng, X. α-Cyanostilbene and fluorene based bolaamphiphiles: Synthesis, self-assembly, and AIEE properties with potential as white-light emissive materials and light-emitting liquid crystal displays. J. Mater. Chem. C 2020, 8, 17474–17481. [Google Scholar] [CrossRef]
- Fan, H.; Li, K.; Tu, T.; Zhu, X.; Zhang, L.; Liu, M. ATP-induced emergent circularly polarized luminescence and encryption. Angew. Chem. Int. Ed. 2022, 61, e202200727. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, L.; Chen, W.; Ji, L.; Zhang, L.; Lu, W.; Fang, Z.; Guo, F.; Qi, L.; Liu, M. Helically grooved gold nanoarrows:controlled fabrication, superhelix, and transcribed chiroptical switching. CCS Chem. 2020, 2, 2473–2484. [Google Scholar]
- Jiang, H.; Jiang, Y.; Han, J.; Zhang, L.; Liu, M. Helical nanostructures: Chirality transfer and a photodriven transformation from superhelix to nanokebab. Angew. Chem. Int. Ed. 2019, 58, 785–790. [Google Scholar] [CrossRef]
- Han, W.; Xiang, W.; Li, Q.; Zhang, H.; Yang, Y.; Shi, J.; Ji, Y.; Wang, S.; Ji, X.; Khashab, N.M.; et al. Water compatible supramolecular polymers: Recent progress. Chem. Soc. Rev. 2021, 50, 10025–10043. [Google Scholar] [CrossRef]
- Babu, S.S.; Praveen, V.K.; Ajayaghosh, A. Functional π-gelators and their applications. Chem. Rev. 2014, 114, 1973–2129. [Google Scholar] [CrossRef]
- Xia, D.; Wang, P.; Ji, X.; Niveen Khashab, M.; Sessler, J.L.; Huang, F. Functional supramolecular polymeric networks: The marriage of covalent polymers and macrocycle-based host–guest interactions. Chem. Rev. 2020, 120, 6070–6123. [Google Scholar] [CrossRef]
- Verma, P.; Rahimi, F.A.; Samanta, D.; Kundu, A.; Dasgupta, J.; Maji, T.K. Visible-light-driven photocatalytic CO2 reduction to CO/CH4 using a metal–organic “soft” coordination polymer gel. Angew. Chem. Int. Ed. 2022. [Google Scholar] [CrossRef]
- Hu, Y.; Gao, S.; Lu, H.; Ying, J.Y. Acid-resistant and physiological pH-responsive DNA hydrogel composed of a-motif and i-motif toward oral insulin delivery. J. Am. Chem. Soc. 2022, 144, 5461–5470. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, S.; Ooi, H.W.; Suylen, D.; Duimel, H.; Hackeng, T.M.; Blitterswijk, C.V.; Baker, M.B. Desymmetrization via activated esters enables rapid synthesis of multifunctional benzene-1,3,5-tricarboxamides and creation of supramolecular hydrogelators. J. Am. Chem. Soc. 2022, 144, 4057–4070. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, T.; Miao, L.; Kaur, P.; Men, S.; Wang, Q.; Gong, X.; Fang, Y.; Zhai, C.; Zhang, S.; et al. A highly sensitive and ultra-stretchable zwitterionic liquid hydrogel-based sensor as anti-freezing ionic skin. J. Mater. Chem. A 2022, 10, 3970–3988. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, X.; Ding, J.; Huang, B.; Wang, P.; Zhao, Y.; Mu, Q.; Zhang, S.; Ren, C.; Xu, W. Hydrogels for underwater adhesion: Adhesion mechanism, design strategies and applications. J. Mater. Chem. A 2022, 10, 11823–11853. [Google Scholar] [CrossRef]
- Xie, X.-Q.; Zhang, Y.; Laing, Y.; Wang, M.; Cui, Y.; Li, J.; Liu, C.-S. Programmable transient supramolecular chiral G-quadruplex hydrogels by a chemically fueled non-equilibrium self-assembly strategy. Angew. Chem. Int. Ed. 2022, 61, e202114471. [Google Scholar] [CrossRef]
- Guo, Y.; Bae, J.; Fang, Z.; Li, P.; Zhao, F.; Yu, G. Hydrogels and hydrogel-derived materials for energy and water sustainability. Chem. Rev. 2020, 120, 7642–7707. [Google Scholar] [CrossRef]
- Cao, X.; Gao, A.; Hou, J.-T.; Yi, T. Fluorescent supramolecular self-assembly gels and their application as sensors: A review. Coord. Chem. Rev. 2021, 434, 213792. [Google Scholar] [CrossRef]
- Wang, T.; Ménard-Moyon, C.; Bianco, A. Self-assembly of amphiphilic amino acid derivatives for biomedical applications. Chem. Soc. Rev. 2022, 51, 3535–3560. [Google Scholar] [CrossRef]
- Lou, X.-Y.; Song, N.; Yang, Y.-W. A stimuli-responsive pillar[5]arene-based hybrid material with enhanced tunable multicolor luminescence and ion-sensing ability. Nat. Sci. Rev. 2021, 8, nwaa281. [Google Scholar] [CrossRef]
- Sun, Y.; Le, X.; Zhou, S.; Chen, T. Recent progress in smart polymeric gel-based information storage for anti-counterfeiting. Adv. Mater. 2022. [Google Scholar] [CrossRef]
- Kuosmanen, R.; Rissanen, K.; Sievänen, E. Steroidal supramolecular metallogels. Chem. Soc. Rev. 2020, 49, 1977–1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correa, S.; Grosskopf, A.K.; Hernandez, H.L.; Chan, D.; Yu, A.C.; Stapleton, L.M.; Appel, E.A. Translational applications of hydrogels. Chem. Rev. 2021, 121, 11385–11457. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, Z.; Chu, C.; Ni, Y.; Neisiany, R.E.; You, Z. Biodegradable elastomers and gels for elastic electronics. Adv. Sci. 2022, 9, 2105146. [Google Scholar] [CrossRef]
- Yin, G.; Huang, J.; Liu, D.; Li, R.; Wei, S.; Si, M.; Ni, F.; Zheng, Y.; Yang, Q.; Zhou, R.; et al. Mechanochemical transformation of fluorescent hydrogel based on dynamic lanthanide-terpyridine coordination. Chin. Chem. Lett. 2020, in press. [Google Scholar] [CrossRef]
- Shen, F.; Wang, T.; Yu, X.; Li, Y. Free radical oxidation reaction for selectively solvatochromic sensors with dynamic sensing ability. Chin. Chem. Lett. 2020, 31, 1919–1922. [Google Scholar] [CrossRef]
- Zhao, Q.; Gong, G.-F.; Yang, H.-L.; Zhang, Q.-P.; Yao, H.; Zhang, Y.-M.; Lin, Q.; Qu, W.-J.; Wei, T.-B. Pillar[5]arene-based supramolecular AIE hydrogel with white light emission for ultrasensitive detection and effective separation of multianalytes. Polym. Chem. 2020, 11, 5455–5462. [Google Scholar] [CrossRef]
- Cao, X.; Li, Y.; Gao, A.; Yu, Y.; Zhou, Q.; Chang, X.; Hei, X. Multifunctional fluorescent naphthalimide selfassembly system for the detection of Cu2+ and K+ and continuous sensing of organic amines and gaseous acids. J. Mater. Chem. C 2019, 7, 10589–10597. [Google Scholar] [CrossRef]
- Cao, X.; Li, Y.; Yu, Y.; Fu, S.; Gao, A.; Chang, X. Multifunctional supramolecular self-assembly system for colorimetric detection of Hg2+, Fe3+, Cu2+ and continuous sensing of volatile acids and organic amine gases. Nanoscale 2019, 11, 10911–10920. [Google Scholar] [CrossRef]
- Cao, X.; Zhao, N.; Gao, A.; Ding, Q.; Li, Y.; Chang, X. Terminal molecular isomer-effect on supramolecular self-assembly system based on naphthalimide derivative and its sensing application for mercury(II) and iron(III) ions. Langmuir 2018, 34, 7404–7415. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, F.; Zhang, Y.; Peng, F.; Huang, Z.; Zhang, W.; Zhao, W. A dual-mode turn-on fluorescent BODIPY-based probe for visualization of mercury ions in living cells. Analyst 2016, 141, 4789–4795. [Google Scholar] [CrossRef]
- Boening, D.W. Ecological effects, transport, and fate of mercury: A general review. Chemosphere 2000, 40, 1335–1351. [Google Scholar] [CrossRef]
- Dai, D.; Li, Z.; Yang, J.; Wang, C.; Wu, J.-R.; Wang, Y.; Zhang, D.; Yang, Y.-W. Supramolecular assembly-induced emission enhancement for efficient mercury(ii) detection and removal. J. Am. Chem. Soc. 2019, 141, 4756–4763. [Google Scholar] [CrossRef] [PubMed]
- Aragay, G.; Pons, J.; Merkoçi, A. Recent trends in macro-, micro-, and nanomaterial-based tools and strategies for heavy-metal detection. Chem. Rev. 2011, 111, 3433–3458. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Y.; Li, Z.; Li, K.; Yu, X.-Q. Small molecular fluorescent probes for the detection of lead, cadmium and mercury ions. Coord. Chem. Rev. 2021, 429, 213691. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Y.-N.; Dong, W.-W.; Wu, Y.-P.; Li, D.-S.; Zhang, Q.-C. A robust luminescent Tb(III)-MOF with lewis basic pyridyl sites for the highly sensitive detection of metal ions and small molecules. Inorg. Chem. 2016, 55, 3265–3271. [Google Scholar] [CrossRef]
- Roy, S.G.; Kumar, A.; Misra, N.; Ghosh, K. Pyridoxal-based low molecular weight progelator as a new chemosensor for the recognition of Ag+ and Hg2+ under different conditions. Mater. Adv. 2022, 3, 5836–5844. [Google Scholar] [CrossRef]
- Revesz, I.A.; Hickey, S.M.; Sweetman, M.J. Metal ion sensing with graphene quantum dots: Detection of harmful contaminants and biorelevant species. J. Mater. Chem. B 2022, 10, 4346–4362. [Google Scholar] [CrossRef]
- Yan, X.; Li, P.; Song, X.; Li, J.; Ren, B.; Gao, S.; Cao, R. Recent progress in the removal of mercury ions from water based MOFs materials. Coord. Chem. Rev. 2021, 443, 214034. [Google Scholar] [CrossRef]
- Li, S.; Zhang, C.; Wang, S.; Liu, Q.; Feng, H.; Ma, X.; Guo, J. Electrochemical microfluidics techniques for heavy metal ion detection. Analyst 2018, 143, 4230–4246. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Shang, N.; Zhu, Q.; Yan, L.; Liu, C. Construction of fluorescence resonance energy transfer system using oxidized carbon nanoparticles as acceptors and the application of mercury ion detection. J. Xinyang Norm. Univ. 2020, 33, 630–634. [Google Scholar]
- Wang, H.; Bai, H.; Mao, A.; Dong, G. Glutathione stabilized copper nanoclusters based fluorescent method for the sensitive detection of Hg2+ ion. J. Xinyang Norm. Univ. (Nat. Sci. Ed.) 2019, 32, 600–603. [Google Scholar]
- Guan, X.; Wang, L.; Liu, M.; Wang, K.; Yang, X.; Ding, Y.; Tong, J.; Lei, Z.; Lai, S. A versatile synthetic approach to tunable dual-emissive Pdots with very small-size based on amphiphilic block copolymers for cell imaging. Mater. Chem. Front. 2021, 5, 355–367. [Google Scholar] [CrossRef]
- Zhang, F.; Xie, H.; Guo, B.; Zhu, C.; Xu, J. AIE-active macromolecules: Designs, performances, and applications. Polym. Chem. 2022, 13, 8–43. [Google Scholar] [CrossRef]
- Rouhani, F.; Morsali, A.; Retailleau, P. Simple one-pot preparation of a rapid response aie fluorescent metal-organic framework. ACS Appl. Mater. Interf. 2018, 10, 36259–36266. [Google Scholar] [CrossRef]
- Li, H.-Y.; Zhao, S.-N.; Zang, S.-Q.; Li, J. Functional metal–organic frameworks as effective sensors of gases and volatile compounds. Chem. Soc. Rev. 2020, 49, 6364–6401. [Google Scholar] [CrossRef]
- Asad, M.; Anwara, I.M.; Abbas, A.; Younas, A.; Hussain, S.; Gao, R.; Li, L.-K.; Shahid, M.; Khan, S. AIE based luminescent porous materials as cutting-edge tool for environmental monitoring: State of the art advances and perspectives. Coord. Chem. Rev. 2022, 463, 214539. [Google Scholar] [CrossRef]
- Gao, A.; Han, Q.; Wang, Q.; Cao, X. Synthesis and properties study of bis-pyridine derivative gel system. J. Xinyang Norm. Univ. 2021, 34, 614–618. [Google Scholar]
- Khuphe, M.; Mukonoweshuro, B.; Kazlauciunas, A.; Thornton, P.D. A vegetable oil-based organogel for use in pH-mediated drug delivery. Soft Matter 2015, 11, 9160–9167. [Google Scholar] [CrossRef]
- Reja, S.I.; Khan, I.A.; Bhalla, V.; Kumar, M. A TICT based NIR-fluorescent probe for human serum albumin: A pre-clinical diagnosis in blood serum. Chem. Commun. 2016, 52, 1182–1185. [Google Scholar] [CrossRef]
- Tao, X.; Chen, X.; Chen, T.; Du, G.; Wang, Y.; Li, Q. Building multi-color emitters with tailored lanthanide-based supramolecular metallogels. Colloid Surf. A 2022, 634, 127910. [Google Scholar]
- Wang, Y.; Niu, D.; Ouyang, G.; Liu, M. Double helical π-aggregate nanoarchitectonics for amplified circularly polarized luminescence. Nat. Commun. 2022, 13, 1710. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.; Zhang, X.; Wang, Y.; Shao, C.; Shang, L.; Zhao, Y. Bio-inspired wettability patterns for biomedical applications. Mater. Horiz. 2021, 8, 124–144. [Google Scholar] [CrossRef] [PubMed]
- Shome, A.; Das, A.; Borbora, A.; Dhar, M.; Manna, U. Role of chemistry in bio-inspired liquid wettability. Chem. Soc. Rev. 2022, 51, 5452–5497. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.; Cao, C.; Huang, J.; Zhang, X.; Tang, Y.; Zhou, X.; Zhang, K.; Chen, Z.; Lai, Y. Rational design of materials interface at nanoscale towards intelligent oil–water separation. Nanoscale Horiz. 2018, 3, 235–260. [Google Scholar] [CrossRef]
- Lin, Q.; Lu, T.-T.; Lou, J.-C.; Wu, G.-Y.; Wei, T.-B.; Zhang, Y.-M. A “keto–enol tautomerization”-based response mechanism: A novel approach to stimuli-responsive supramolecular gel. Chem. Commun. 2015, 51, 12224–12227. [Google Scholar] [CrossRef]
- Chen, J.-F.; Lin, Q.; Yao, H.; Zhang, Y.-M.; Wei, T.-B. Pillar[5]arene-based multifunctional supramolecular hydrogel: Multistimuli responsiveness, self-healing, fluorescence sensing, and conductivity. Mater. Chem. Front. 2018, 2, 999–1003. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Li, Y.-J.; Wang, Z.-H.; Sun, X.-W.; Yang, Q.-Y.; Dong, H.-Q.; Zhang, Y.-M.; Wei, T.-B.; Yao, H.; Lin, Q. A mechanically self-locked gemini-[1]rotaxane-assembled microsphere and its properties on L-Arg controlled reversible morphology and fluorescence changes. J. Mater. Chem. C 2021, 9, 10347–10353. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, H.; Gao, A.; Ge, X.; Chang, X.; Cao, X. Bis-naphthalimide-based supramolecular self-assembly system for selective and colorimetric detection of oxalyl chloride and phosgene in solution and gas phase. Chin. Chem. Lett. 2022, in press. [Google Scholar] [CrossRef]
- Yang, H.-L.; Sun, X.-W.; Zhang, Y.-M.; Wang, Z.-H.; Zhu, W.; Fan, Y.-Q.; Wei, T.-B.; Yao, H.; Lin, Q. A bi-component supramolecular gel for selective fluorescence detection and removal of Hg2+ in water. Soft Matter 2019, 15, 9547–9552. [Google Scholar] [CrossRef]












| Solvents | 1 | Solvents | 1 |
|---|---|---|---|
| n-hexane | I | 1, 4-dioxane | P |
| toluene | P | ethyl acetate | G(25) |
| methanol | G(25) | petroleum ether | I |
| ethanol | G(25) | DMSO/H2O = 4:1(v/v) | G(6.67) |
| acetonitrile | G(25) | DMF/H2O = 4:1(v/v) | G(20) |
| acetone | I | THF | S |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, A.; Han, Q.; Wang, Q.; Wan, R.; Wu, H.; Cao, X. Bis-Pyridine-Based Organogel with AIE Effect and Sensing Performance towards Hg2+. Gels 2022, 8, 464. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8080464
Gao A, Han Q, Wang Q, Wan R, Wu H, Cao X. Bis-Pyridine-Based Organogel with AIE Effect and Sensing Performance towards Hg2+. Gels. 2022; 8(8):464. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8080464
Chicago/Turabian StyleGao, Aiping, Qingqing Han, Qingqing Wang, Rong Wan, Huijuan Wu, and Xinhua Cao. 2022. "Bis-Pyridine-Based Organogel with AIE Effect and Sensing Performance towards Hg2+" Gels 8, no. 8: 464. https://0-doi-org.brum.beds.ac.uk/10.3390/gels8080464