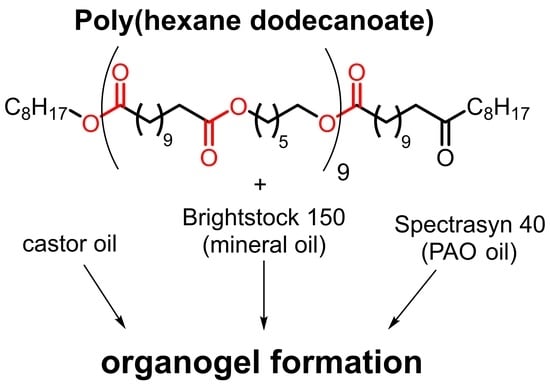
Comparative Studies on the Organogel Formation of a Polyester in Three Different Base Oils by X-ray Analysis, Rheology and Infrared Spectroscopy
Abstract
:1. Introduction
2. Results and Discussion
2.1. Gel Characterisation
2.1.1. Tube Inversion Test
2.1.2. X-ray Scattering
2.1.3. Infrared Spectroscopy (IR)
2.1.4. Rheology
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Polyester Synthesis
4.3. Tube Inversion Test
4.4. X-ray Scattering
4.5. Infrared Spectroscopy
4.6. Rheology
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saveliev, Y.V.; Savelieva, N.N. Automation of industrial processes and everyday life. IOP Conf. Ser. Mater. Sci. Eng. 2019, 663, 012068. [Google Scholar] [CrossRef]
- Lakes, S.C. Automotove Gear Lubricants. In Synthetics, Mineral Oils, and Bio-Based Lubricants: Chemsitry and Technology, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2020; pp. 557–571. [Google Scholar]
- Martini, A.; Dong, A.E.; Ae, Z.; Wang, Q. Reduction in Mixed Lubrication. Tribol. Lett. 2007, 28, 139–147. [Google Scholar] [CrossRef]
- Salih, N.; Salimon, J. A Review on one Trends, Challenges and Prospects, of Ecofriendly Friendly Green-Food Grade Biolubricants. Biointerface Res. Appl. Chem. 2022, 12, 1185–1207. [Google Scholar]
- Domínguez, G.; Blánquez, A.; Borrero-López, A.M.; Valencia, C.; Eugenio, M.E.; Arias, M.E.; Rodríguez, J.; Hernández, M. Eco-Friendly Oleogels from Functionalized Kraft Lignin with Laccase SilA from Streptomyces ipomoeae: An Opportunity to Replace Commercial Lubricants. ACS Sustain. Chem. Eng. 2021, 9, 4611–4616. [Google Scholar] [CrossRef]
- DeLaurentis, N.; Kadiric, A.; Lugt, P.; Cann, P. The influence of bearing grease composition on friction in rolling/sliding concetrated contacts. Tribol. Int. 2016, 94, 624–632. [Google Scholar] [CrossRef]
- Fan, X.; Li, W.; Li, H.; Zhu, M.; Xia, Y.; Wang, J. Probing the effect of thickener on tribological properties of lubricating greases. Tribol. Int. 2018, 118, 128–139. [Google Scholar] [CrossRef]
- Syahir, A.Z.; Zulkifli, N.W.M.; Masjuki, H.H.; Kalam, M.A.; Alabdulkarem, A.; Gulzar, M.; Khuong, L.S.; Harith, M.H. A review on bio-based lubricants and their applications. J. Clean. Prod. 2017, 168, 997–1016. [Google Scholar] [CrossRef]
- Kumar Jain, A.; Suhane, A. Capability of Biolubricants as Alternative Lubricant in Industrial and Maintenance Applications. Int. J. Curr. Eng. Technol. 2013, 3, 179–183. [Google Scholar]
- Vafaei, S.; Fischer, D.; Jopen, M.; Jacobs, G.; Koenig, F.; Weberskirch, R. Investigation of Tribological Behavior of Lubricatin Greases Composed of Different Bio-Based Polymer Thickeners. Lubricants 2021, 9, 80. [Google Scholar] [CrossRef]
- Zhou, C.; Wei, Z.; Yu, Y.; Shao, S.; Leng, X.; Wang, Y.; Li, Y. Biobased long-chain aliphatic polyesters of 1,12-dodecanedioic acid with a variety of diols: Odd-even effect and mechanical properties. Mater. Today Commun. 2019, 19, 450–458. [Google Scholar] [CrossRef]
- Vafaei, S.; Jopen, M.; Jacobs, G.; König, F.; Weberskirch, R. Synthesis and tribological behavior of bio-based lubrication greases with bio-based polyester thickener systems. J. Clean. Prod. 2022, 364, 132659. [Google Scholar] [CrossRef]
- Gallego, R.; Cidade, T.; Sánchez, R.; Valencia, C.; Franco, J.M. Tribological behaviour of novel chemically modified iopolymer-thickened lubricating greases investigated in a steel–steel rotating ball-on-three plates tribology cell. Tribol. Int. 2016, 94, 652–660. [Google Scholar] [CrossRef]
- Rodríguez-de Léon, E.; Bah, M.; Báez, J.E.; Hernández-Sierra, M.T.; Moreno, K.J.; Nunez-Vilchis, A.; Bonilla-Cruz, J.; Shea, K.J. Sustainable xanthophylls-containing poly(3-caprolactone)s: Synthesis, characterization, and use in green lubricants. RSC Adv. 2022, 12, 30851–30859. [Google Scholar] [CrossRef]
- Kuratomi, T.; Nagano, K. Lubricant base oil. Int. J. Pharm. 2009, 56, 1986–2001. [Google Scholar]
- Kang, J.; Gi, H.; Choe, R.; Yun, S.I. Fabrication and characterization of poly(3-hydroxybutyrate) gels using non-solvent-induced phase separation. Polymer 2016, 104, 61–71. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Che, X.; Yu, L.; Jia, W.; Shen, R.; Chen, J.; Ma, Y.; Chen, G.Q. Synthesis and Characterization of Polyhydroxyalkanoate Organo/Hydrogels. Biomacromolecules 2019, 20, 3303–3312. [Google Scholar] [CrossRef] [PubMed]
- Shikata, T.; Nishida, T.; Isare, B.; Linares, M.; Lazzaroni, R.; Bouteiller, L. Structure and Dynamics of a Bisurea-Based Supramolecular Polymer in n-Dodecane. J. Phys. Chem. B 2008, 112, 8459–8465. [Google Scholar] [CrossRef] [PubMed]
- Ouhib, F.; Raynal, M.; Jouvelet, B.; Isare, B.; Bouteiller, L. Hydrogen bonded supramolecular polymers in moderately polar solvents. Chem. Commun. 2011, 47, 10683–10685. [Google Scholar] [CrossRef]
- Suzuki, M.; Setoguchi, C.; Shirai, H.; Hanabusa, K. Organogelation by Polymer Organogelators with a L-Lysine Derivative: Formation of a Three-Dimensional Network Consisting of Supramolecular and Conventional Polymers. Chem. Eur. J. 2007, 13, 8193–8200. [Google Scholar] [CrossRef] [PubMed]
- Lyadova, A.S.; Yarmusha, Y.M.; Parenagoa, O.P. Colloidal Stability of Greases Based on Oils with Organic Thickening Agents. Russ. J. Appl. Chem. 2019, 92, 1805–1809. [Google Scholar] [CrossRef]
- Yang, H.K.; Zhang, C.; He, X.N.; Wang, P.Y. Effects of alkyl chain lengths on 12-hydroxystearic acid derivatives based supramolecular organogels. Colloids Surf. A 2021, 616, 126319. [Google Scholar] [CrossRef]
- Babu, S.S.; Praveen, V.K.; Ajayaghosh, A. Functional _π-Gelators and Their Applications. Chem. Rev. 2014, 114, 1973–2129. [Google Scholar] [CrossRef] [PubMed]
- Guenet, J.M. Physical Aspects of Organogelation: A Point of View. Gels 2021, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Singh, W.P.; Bhandari, S.; Singh, R.S. Organogelators derived from the bisphenol A scaffold. New J. Chem. 2021, 45, 15655–15666. [Google Scholar] [CrossRef]
- Damavandi, F.; Soares, J.B.P. Facile and Efficient Phase-Selective Powder Polymer Organogelator for Oil Spill Remediation. Langmuir 2022, 38, 12666–12673. [Google Scholar] [CrossRef]
- Nandi, M.; Maiti, B.; Banerjee, S.; De, P. Hydrogen Bonding Driven Self-Assembly of Side-Chain Amino Acid and Fatty Acid Appended Poly(methacrylate)s: Gelation and Application in Oil Spill Recovery. J. Polym. Sci. A Polym. Chem. 2019, 57, 511–521. [Google Scholar] [CrossRef]
- Hammersley, A.P. ESRF Internal Report, ESRF98HA01T, FIT2D V9.129 Reference Manual V3.1 (1998). Available online: https://www.esrf.fr/computing/scientific/FIT2D/FIT2D_REF/node268.html (accessed on 15 August 2023).
- Böhmer, R.; Gainaru, C.; Richert, R. Structure and dynamics of monohydroxy alcohols—Milestones towards their microscopic understanding, 100 years after Debye. Phys. Rep. 2014, 545, 125–195. [Google Scholar] [CrossRef]
- Pozar, M.; Bolle, J.; Sternemann, C.; Perera, A. On the X-ray Scattering Pre-peak of Linear Mono-ols and the Related Microstructure from Computer Simulations. J. Phys. Chem. B 2020, 124, 8358–8371. [Google Scholar] [CrossRef]
- Bolle, J.; Bierwirth, S.P.; Požar, M.; Perera, A.; Paulus, M.; Münzner, P.; Albers, C.; Dogan, S.; Elbers, M.; Sakrowski, R.; et al. Isomeric effects in structure formation and dielectric dynamics of different octanols. Phys. Chem. Chem. Phys. 2021, 23, 24211–24221. [Google Scholar] [CrossRef]
- Pinault, T.; Isare, B.; Bouteiller, L. Solvents with Similar Bulk Properties Induce Distinct Supramolecular Architectures. ChemPhysChem 2006, 7, 816–819. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Bo, S.S.; Wang, R.; Fang, J.H.; Wang, X.G.; Bai, Y.Y.; Ma, Z.F.; Liang, Y.J.; Zhang, M.; Yu, Q.L.; et al. Supramolecular Polymer Gel Lubricant with Excellent Mechanical Stability and Tribological Performances. ACS Appl. Mater. Interfaces 2022, 14, 45934–45944. [Google Scholar] [CrossRef]
- Genio, F.A.F.; Paderes, M.C. Functional Supramolecular Gels Comprised of Bis-Urea Compounds and Cosmetic Solvents. Chem. Sel. 2021, 6, 7906–7911. [Google Scholar] [CrossRef]
- Hill, J.P.; Jin, W.; Kosaka, A.; Fukushima, T.; Ichihara, H.; Shimomura, T.; Ito, K.; Hashizume, T.; Ishii, N.; Aida, T. Self-Assembled Hexa-peri-hexabenzocoronene Graphitic Nanotube. Science 2004, 304, 1481–1483. [Google Scholar] [CrossRef] [PubMed]
- Mezger, T.G. Das Rheologie Handbuch: Für Anwender von Rotations- Und Oszillations-Rheometern; Vincentz: Hannover, Germany, 2006. [Google Scholar]
- Böhme, G. Strömungsmechanik Nichtnewtonscher Fluide; Teubner: Leipzig, Germany, 2000. [Google Scholar]
- Jopen, M.; Degen, P.; Henzler, S.; Grabe, B.; Hiller, W.; Weberskirch, R. Polyurea Thickened Lubricating Grease—The Effect of Degree of Polymerization on Rheological and Tribological Properties. Polymers 2022, 14, 795. [Google Scholar] [CrossRef] [PubMed]
- Carothers, W.H. Polymers and polyfunctionality. Trans. Faraday Soc. 1936, 32, 39–49. [Google Scholar] [CrossRef]
- Prabhu, R.; Yelamaggad, C.V.; Shanker, G. Self-organisation properties of homomeric dipeptides derived from valine. Liq. Cryst. 2014, 41, 1008–1016. [Google Scholar] [CrossRef]
- Bouteiller, L.; Colombani, O. Thickness Transition of a Rigid Supramolecular Polymer. J. Am. Chem. Soc. 2005, 127, 8893–8898. [Google Scholar] [CrossRef]
- Krywka, C.; Paulus, M.; Sternemann, C.; Volmer, M.; Remhof, A.; Nowak, G.; Nefedov, A.; Pöter, B.; Spiegel, M.; Tolan, M. The new diffractometer for surface X-ray diffraction at beamline BL9 of DELTA. J. Synchrotron Rad. 2006, 13, 8–13. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jopen, M.; Paulus, M.; Sternemann, C.; Degen, P.; Weberskirch, R. Comparative Studies on the Organogel Formation of a Polyester in Three Different Base Oils by X-ray Analysis, Rheology and Infrared Spectroscopy. Gels 2023, 9, 696. https://0-doi-org.brum.beds.ac.uk/10.3390/gels9090696
Jopen M, Paulus M, Sternemann C, Degen P, Weberskirch R. Comparative Studies on the Organogel Formation of a Polyester in Three Different Base Oils by X-ray Analysis, Rheology and Infrared Spectroscopy. Gels. 2023; 9(9):696. https://0-doi-org.brum.beds.ac.uk/10.3390/gels9090696
Chicago/Turabian StyleJopen, Max, Michael Paulus, Christian Sternemann, Patrick Degen, and Ralf Weberskirch. 2023. "Comparative Studies on the Organogel Formation of a Polyester in Three Different Base Oils by X-ray Analysis, Rheology and Infrared Spectroscopy" Gels 9, no. 9: 696. https://0-doi-org.brum.beds.ac.uk/10.3390/gels9090696