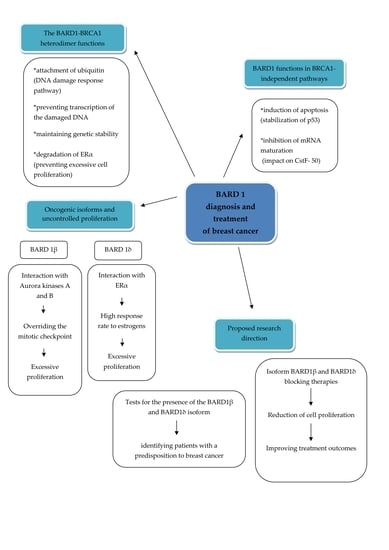
BARD1 and Breast Cancer: The Possibility of Creating Screening Tests and New Preventive and Therapeutic Pathways for Predisposed Women
Abstract
:1. Introduction
2. Scope of the Review
3. The Results of the Review on the Topics Covered
3.1. The Significance of BARD1 in Genetic Predisposition to Breast Cancer
3.2. Utility of BARD1 in Surveillance Programs
3.3. BARD1 Gene as a Potential Target of New Anticancer Therapies Including Sensitivity to Chemotherapy with a Focus on Breast Cancer
3.4. BARD1 Gene Alterations in Neoadjuvant Setting in Breast Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, L.C.; Wang, Z.W.; Tsan, J.T.; Spillman, M.A.; Phung, A.; Xu, X.L.; Yang, M.C.; Hwang, L.Y.; Bowcock, A.M.; Baer, R. Identification of a RING protein that can interact in vivo with the BRCA1 gene product. Nat. Genet. 1996, 14, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Gronwald, J.; Jauch, A.; Cybulski, C.; Schoell, B.; Böhm-Steuer, B.; Lener, M.; Grabowska, E.; Górski, B.; Jakubowska, A.; Domagała, W.; et al. Comparison of genomic abnormalities between BRCAX and sporadic breast cancers studied by comparative genomic hybridization. Int. J. Cancer 2005, 114, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Meza, J.E.; Brzovic, P.S.; King, M.C.; Klevit, R.E. Mapping the functional domains of BRCA1. Interaction of the ring finger domains of BRCA1 and BARD1. J. Biol. Chem. 1999, 274, 5659–5665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irminger-Finger, I.; Ratajska, M.; Pilyugin, M. New concepts on BARD1: Regulator of BRCA pathways and beyond. Int. J. Biochem. Cell Biol. 2016, 72, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Yu, X. Function of BRCA1 in the DNA damage response is mediated by ADP-ribosylation. Cancer Cell 2013, 23, 693–704. [Google Scholar] [CrossRef] [Green Version]
- Fleck, O.; Nielsen, O. DNA repair. J. Cell Sci. 2004, 117, 515–517. [Google Scholar] [CrossRef] [Green Version]
- Kleiman, F.E.; Wu-Baer, F.; Fonseca, D.; Kaneko, S.; Baer, R.; Manley, J.L. BRCA1/BARD1 inhibition of mRNA 3’ processing involves targeted degradation of RNA polymerase II. Genes Dev. 2005, 19, 1227–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dizin, E.; Irminger-Finger, I. Negative feedback loop of BRCA1-BARD1 ubiquitin ligase on estrogen receptor alpha stability and activity antagonized by cancer-associated isoform of BARD1. Int. J. Biochem. Cell Biol. 2010, 42, 693–700. [Google Scholar] [CrossRef]
- Feki, A.; Jefford, C.E.; Berardi, P.; Wu, J.Y.; Cartier, L.; Krause, K.H.; Irminger-Finger, I. BARD1 induces apoptosis by catalysing phosphorylation of p53 by DNA-damage response kinase. Oncogene 2005, 24, 3726–3736. [Google Scholar] [CrossRef] [Green Version]
- Kleiman, F.E.; Manley, J.L. Functional interaction of BRCA1-associated BARD1 with polyadenylation factor CstF-50. Science 1999, 285, 1576–1579. [Google Scholar] [CrossRef]
- Bosse, K.R.; Diskin, S.J.; Cole, K.A. Common variation at BARD1 results in the expression of an oncogenic isoform that influences neuroblastoma susceptibility and oncogenicity. Cancer Res. 2012, 72, 2068–2078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melchor, L.; Benítez, J. The complex genetic landscape of familial breast cancer. Hum. Genet. 2013, 132, 845–863. [Google Scholar] [CrossRef] [PubMed]
- Klonowska, K.; Ratajska, M.; Czubak, K.; Kuzniacka, A.; Brozek, I.; Koczkowska, M.; Sniadecki, M.; Debniak, J.; Wydra, D.; Balut, M.; et al. Analysis of large mutations in BARD1 in patients with breast and/or ovarian cancer: The Polish population as an example. Sci. Rep. 2015, 5, 10424. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, Y.; Zhang, C.; Chu, J.; Wu, Y.; Li, Y.; Liu, J.; Li, Q.; Li, S.; Shi, Q.; et al. Tamoxifen-resistant breast cancer cells are resistant to DNA-damaging chemotherapy because of upregulated BARD1 and BRCA1. Nat. Commun. 2018, 9, 1595. [Google Scholar] [CrossRef] [PubMed]
- Klonowska, K.; Ratajska, M.; Wojciechowska, M.; Kozlowski, P. Genetic predisposition to breast and/or ovarian cancer—Ocus on the candidate BARD1. Comput. Biol. Bionanotechnol. 2014, 95, 2013–2014. [Google Scholar]
- Beggs, A.D.; Hodgson, S.V. Genomics and breast cancer: The different levels of inherited susceptibility. Eur. J. Hum. Genet. 2009, 17, 855–856. [Google Scholar] [CrossRef] [Green Version]
- Antoniou, A.; Pharoah, P.D.; Narod, S.; Risch, H.A.; Eyfjord, J.E.; Hopper, J.L.; Loman, N.; Olsson, H.; Johannsson, O.; Borg, A.; et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: A combined analysis of 22 studies. Am. J. Hum. Genet. 2003, 72, 1117–1130. [Google Scholar] [CrossRef] [Green Version]
- Schon, K.; Tischkowitz, M. Clinical implications of germline mutations in breast cancer: TP53. Breast Cancer Res. Treat. 2018, 167, 417–423. [Google Scholar] [CrossRef] [Green Version]
- Angeli, D.; Salvi, S.; Tedaldi, G. Genetic Predisposition to Breast and Ovarian Cancers: How Many and Which Genes to Test? Int. J. Mol. Sci. 2020, 21, 1128. [Google Scholar] [CrossRef] [Green Version]
- Couch, F.J.; Shimelis, H.; Hu, C.; Hart, S.N.; Polley, E.C.; Na, J.; Hallberg, E.; Moore, R.; Thomas, A.; Lilyquist, J.; et al. Associations Between Cancer Predisposition Testing Panel Genes and Breast Cancer. JAMA Oncol. 2017, 3, 1190–1196. [Google Scholar] [CrossRef] [Green Version]
- Shimelis, H.; LaDuca, H.; Hu, C.; Hart, S.N.; Na, J.; Thomas, A.; Akinhanmi, M.; Moore, R.M.; Brauch, H.; Cox, A.; et al. Triple-Negative Breast Cancer Risk Genes Identified by Multigene Hereditary Cancer Panel Testing. J. Natl. Cancer Inst. 2018, 110, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Churpek, J.E.; Walsh, T.; Zheng, Y.; Moton, Z.; Thornton, A.M.; Lee, M.K.; Casadei, S.; Watts, A.; Neistadt, B.; Churpek, M.M.; et al. Inherited predisposition to breast cancer among African American women. Breast Cancer Res. Treat. 2015, 149, 31–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couch, F.J.; Hart, S.N.; Sharma, P.; Toland, A.E.; Wang, X.; Miron, P.; Olson, J.E.; Godwin, A.K.; Pankratz, V.S.; Olswold, C.; et al. Inherited mutations in 17 breast cancer susceptibility genes among a large triple-negative breast cancer cohort unselected for family history of breast cancer. J. Clin. Oncol. 2015, 33, 304–311. [Google Scholar] [CrossRef]
- Park, J.S.; Lee, S.T.; Nam, E.J.; Han, J.W.; Lee, J.Y.; Kim, J.; Kim, T.I.; Park, H.S. Variants of cancer susceptibility genes in Korean BRCA1/2 mutation-negative patients with high risk for hereditary breast cancer. BMC Cancer 2018, 18, 83. [Google Scholar] [CrossRef]
- Karppinen, S.M.; Heikkinen, K.; Rapakko, K.; Winqvist, R. Mutation screening of the BARD1 gene: Evidence for involvement of the Cys557Ser allele in hereditary susceptibility to breast cancer. J. Med Genet. 2004, 41, 114. [Google Scholar] [CrossRef] [Green Version]
- Ghimenti, C.; Sensi, E.; Presciuttini, S.; Brunetti, I.M.; Conte, P.; Bevilacqua, G.; Caligo, M.A. Germline mutations of the BRCA1-associated ring domain (BARD1) gene in breast and breast/ovarian families negative for BRCA1 and BRCA2 alterations. Genes Chromosom. Cancer 2002, 33, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Ratajska, M.; Antoszewska, E.; Piskorz, A.; Brozek, I.; Borg, Å.; Kusmierek, H.; Biernat, W.; Limon, J. Cancer predisposing BARD1 mutations in breast-ovarian cancer families. Breast Cancer Res. Treat. 2012, 131, 89–97. [Google Scholar] [CrossRef]
- Ratajska, M.; Matusiak, M.; Kuzniacka, A.; Wasag, B.; Brozek, I.; Biernat, W.; Koczkowska, M.; Debniak, J.; Sniadecki, M.; Kozlowski, P.; et al. Cancer predisposing BARD1 mutations affect exon skipping and are associated with overexpression of specific BARD1 isoforms. Oncol. Rep. 2015, 34, 2609–2617. [Google Scholar] [CrossRef] [Green Version]
- Suszynska, M.; Kluzniak, W.; Wokolorczyk, D.; Jakubowska, A.; Huzarski, T.; Gronwald, J.; Debniak, T.; Szwiec, M.; Ratajska, M.; Klonowska, K.; et al. BARD1 is A Low/Moderate Breast Cancer Risk Gene: Evidence Based on An Association Study of the Central European p.Q564X Recurrent Mutation. Cancers 2019, 11, 740. [Google Scholar] [CrossRef] [Green Version]
- Weber-Lassalle, N.; Borde, J.; Weber-Lassalle, K.; Horváth, J.; Niederacher, D.; Arnold, N.; Kaulfuß, S.; Ernst, C.; Paul, V.G.; Honisch, E.; et al. Germline loss-of-function variants in the BARD1 gene are associated with early-onset familial breast cancer but not ovarian cancer. Breast Cancer Res. 2019, 21, 55. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Meeks, H.; Feng, B.J.; Healey, S.; Thorne, H.; Makunin, I.; Ellis, J.; Campbell, I.; Southey, M.; Mitchell, G.; et al. Targeted massively parallel sequencing of a panel of putative breast cancer susceptibility genes in a large cohort of multiple-case breast and ovarian cancer families. J. Med Genet. 2016, 53, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Lang, G.T.; Shi, J.X.; Hu, X.; Zhang, C.H.; Shan, L.; Song, C.G.; Zhuang, Z.G.; Cao, A.Y.; Ling, H.; Yu, K.D.; et al. The spectrum of BRCA mutations and characteristics of BRCA-associated breast cancers in China: Screening of 2,991 patients and 1,043 controls by next-generation sequencing. Int. J. Cancer 2017, 141, 129–142. [Google Scholar] [CrossRef] [Green Version]
- Varol, U.; Kucukzeybek, Y.; Alacacioglu, A.; Somali, I.; Altun, Z.; Aktas, S.; Oktay Tarhan, M. BRCA genes: BRCA 1 and BRCA 2. JBUON 2018, 23, 862–866. [Google Scholar] [PubMed]
- Tantawy, S.A.; Abdelbasset, W.K.; Nambi, G.; Kamel, D.M. Comparative Study Between the Effects of Kinesio Taping and Pressure Garment on Secondary Upper Extremity Lymphedema and Quality of Life Following Mastectomy: A Randomized Controlled Trial. Integr. Cancer Ther. 2019, 18, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanisz, M.; Panczyk, M.; Kurzawa, R.; Grochans, E. The Effect of Prophylactic Adnexectomy on the Quality of Life and Psychosocial Functioning of Women with the BRCA1/BRCA2 Mutations. Int. J. Environ. Res. Public Health 2019, 16, 4995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Weiss, W. Alternative splicing in cancer: Implications for biology and therapy. Oncogene 2015, 34, 1–14. [Google Scholar] [CrossRef]
- Sporn, J.C.; Hothorn, T.; Jung, B. BARD1 expression predicts outcome in colon cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 5451–5462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Y.; Yuan, S.; Chen, X.; Zhu, P.; Li, J.; Qin, L.; Liao, W. Up-regulation of BRCA1-associated RING Domain 1 Promotes Hepatocellular Carcinoma Progression by Targeting Akt Signaling. Sci. Rep. 2017, 7, 7649. [Google Scholar] [CrossRef]
- Li, L.; Ryser, S.; Dizin, E.; Pils, D.; Krainer, M.; Jefford, C.E.; Bertoni, F.; Zeillinger, R.; Irminger-Finger, I. Oncogenic BARD1 isoforms expressed in gynecological cancers. Cancer Res. 2007, 67, 11876–11885. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.Y.; Vlastos, A.T.; Pelte, M.F.; Caligo, M.A.; Bianco, A.; Krause, K.H.; Laurent, G.J.; Irminger-Finger, I. Aberrant expression of BARD1 in breast and ovarian cancers with poor prognosis. Int. J. Cancer 2006, 118, 1215–1226. [Google Scholar] [CrossRef]
- Ryser, S.; Dizin, E.; Jefford, C.E.; Delaval, B.; Gagos, S.; Christodoulidou, A.; Krause, K.-H.; Birnbaum, D.; Irminger-Finger, I. Distinct roles of BARD1 isoforms in mitosis: Full-length BARD1 mediates Aurora B degradation, cancer-associated BARD1beta scaffolds Aurora B and BRCA2. Cancer Res. 2009, 69, 1125–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irminger-Finger, I. BARD1, a possible biomarker for breast and ovarian cancer. Gynecol. Oncol. 2010, 117, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Pilyugin, M.; Descloux, P.; André, P.-A.; Laszlo, V.; Dome, B.; Hegedus, B.; Sardy, S.; Janes, S.; Bianco, A.; Laurent, G.J.; et al. BARD1 serum autoantibodies for the detection of lung cancer. PLoS ONE 2017, 12, e0182356. [Google Scholar] [CrossRef] [Green Version]
- Cimmino, F.; Formicola, D.; Capasso, M. Dualistic Role of BARD1 in Cancer. Genes 2017, 8, 375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hortobagyi, G.N.; Edge, S.B.; Giuliano, A. New and Important Changes in the TNM Staging System for Breast Cancer. Am. Soc. Clin. Oncol. Educ. Book Am. Soc. Clin. Oncol. Annu. Meet. 2018, 38, 457–467. [Google Scholar] [CrossRef]
- Kothari, C.; Diorio, C.; Durocher, F. Gene signatures of breast cancer development and the potential for novel targeted treatments. Pharmacogenomics 2020, 21, 157–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, Z.S.; Ghadamzadeh, M.; Arashloo, F.T.; Amjad, G.; Ebadi, M.R.; Younesi, L. Recent advances of therapeutic targets based on the molecular signature in breast cancer: Genetic mutations and implications for current treatment paradigms. J. Hematol. Oncol. 2019, 12, 38. [Google Scholar] [CrossRef]
- Autier, P.; Boniol, M. Mammography screening: A major issue in medicine. Eur. J. Cancer 2018, 90, 34–62. [Google Scholar] [CrossRef]
- Pinker, K.; Chin, J.; Melsaether, A.N.; Morris, E.A.; Moy, L. Precision Medicine and Radiogenomics in Breast Cancer: New Approaches toward Diagnosis and Treatment. Radiology 2018, 287, 732–747. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Pernas, S.; Tolaney, S.M.; Winer, E.P.; Goel, S. CDK4/6 inhibition in breast cancer: Current practice and future directions. Ther. Adv. Medal Oncol. 2018, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keung, M.Y.; Wu, Y.; Badar, F.; Vadgama, J.V. Response of Breast Cancer Cells to PARP Inhibitors Is Independent of BRCA Status. J. Clin. Med. 2020, 9, 940. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Krais, J.J.; Bernhardy, A.J.; Nicolas, E.; Cai, K.Q.; Harrell, M.I.; Kim, H.H.; George, E.; Swisher, E.M.; Simpkins, F.; et al. RING domain-deficient BRCA1 promotes PARP inhibitor and platinum resistance. J. Clin. Investig. 2016, 126, 3145–3157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledermann, J.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.L.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: A preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014, 15, 852–861. [Google Scholar] [CrossRef]
- Lord, C.J.; Ashworth, A. Mechanisms of resistance to therapies targeting BRCA-mutant cancers. Nat. Med. 2013, 19, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Buys, S.S.; Sandbach, J.F.; Gammon, A.; Patel, G.; Kidd, J.; Brown, K.L.; Sharma, L.; Saam, J.; Lancaster, J.; Daly, M.B. A study of over 35,000 women with breast cancer tested with a 25-gene panel of hereditary cancer genes. Cancer 2017, 123, 1721–1730. [Google Scholar] [CrossRef] [Green Version]
- Lepore, I.; Dell’Aversana, C.; Pilyugin, M.; Conte, M.; Nebbioso, A.; De Bellis, F.; Tambaro, F.P.; Izzo, T.; Garcia-Manero, G.; Ferrara, F.; et al. HDAC inhibitors repress BARD1 isoform expression in acute myeloid leukemia cells via activation of miR-19a and/or b. PLoS ONE 2013, 8, e83018. [Google Scholar] [CrossRef]
- Watanabe, Y.; Maeda, I.; Oikawa, R.; Wu, W.; Tsuchiya, K.; Miyoshi, Y.; Itoh, F.; Tsugawa, K.; Ohta, T. Aberrant DNA methylation status of DNA repair genes in breast cancer treated with neoadjuvant chemotherapy. Genes Cells 2013, 18, 1120–1130. [Google Scholar] [CrossRef] [Green Version]
- González-Rivera, M.; Lobo, M.; López-Tarruella, S.; Jerez, Y.; Del Monte-Millán, M.; Massarrah, T.; Ramos-Medina, R.; Ocaña, I.; Picornell, A.; Santillán Garzón, S.; et al. Frequency of germline DNA genetic findings in an unselected prospective cohort of triple-negative breast cancer patients participating in a platinum-based neoadjuvant chemotherapy trial. Breast Cancer Res. Treat. 2016, 156, 507–515. [Google Scholar] [CrossRef]
- Post, A.; Bussink, J.; Sweep, F.; Span, P.N. Changes in DNA Damage Repair Gene Expression and Cell Cycle Gene Expression Do Not Explain Radioresistance in Tamoxifen-Resistant Breast Cancer. Oncol. Res. 2020, 28, 33–40. [Google Scholar] [CrossRef]
- Ozden, O.; Bishehsari, F.; Bauer, J.; Park, S.H.; Jana, A.; Baik, S.H.; Sporn, J.C.; Staudacher, J.J.; Yazici, C.; Krett, N.; et al. Expression of an Oncogenic BARD1 Splice Variant Impairs Homologous Recombination and Predicts Response to PARP-1 Inhibitor Therapy in Colon Cancer. Sci. Rep. 2016, 6, 26273. [Google Scholar] [CrossRef] [PubMed]

| Level of Predisposition | Gene Penetration | Risk of Breast Cancer | Examples of Affected Genes | Characteristics | Reference |
|---|---|---|---|---|---|
| I | High | High | BRCA1 and BRCA2, TP53, CDH1, STK11, PTEN | Mutations in BRCA1 and BRCA2 are responsible for 16–40% of hereditary breast and ovarian cancers and site-specific breast cancer; inTP53 is associated with up to 85% risk of developing breast cancer by age 60; germline mutations in CDH1 and STK11 are associated with 39–52% and 32–54% risk of developing breast cancer, respectively; germline mutations in the PTEN gene promoter are associated with an 85% lifetime risk of breast cancer | [12,17,18,19] |
| II | Intermediate | Moderate | ATM, CHEK2, BRIP1, BARD1, PALB2 | Mutations in these genes are responsible for a 2- to 4-fold increase in the risk of breast cancer in comparison to population-based risk | [16] |
| III | Low | Low | FGFR2, RAD51 | FGFR2 SNPs increase the risk of breast cancer by increasing the response to estrogen; RAD51 SNP2 i.e., are considered as BRCA1/2 mutation carrier risk modifiers | [13,16] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Śniadecki, M.; Brzeziński, M.; Darecka, K.; Klasa-Mazurkiewicz, D.; Poniewierza, P.; Krzeszowiec, M.; Kmieć, N.; Wydra, D. BARD1 and Breast Cancer: The Possibility of Creating Screening Tests and New Preventive and Therapeutic Pathways for Predisposed Women. Genes 2020, 11, 1251. https://0-doi-org.brum.beds.ac.uk/10.3390/genes11111251
Śniadecki M, Brzeziński M, Darecka K, Klasa-Mazurkiewicz D, Poniewierza P, Krzeszowiec M, Kmieć N, Wydra D. BARD1 and Breast Cancer: The Possibility of Creating Screening Tests and New Preventive and Therapeutic Pathways for Predisposed Women. Genes. 2020; 11(11):1251. https://0-doi-org.brum.beds.ac.uk/10.3390/genes11111251
Chicago/Turabian StyleŚniadecki, Marcin, Michał Brzeziński, Katarzyna Darecka, Dagmara Klasa-Mazurkiewicz, Patryk Poniewierza, Marta Krzeszowiec, Natalia Kmieć, and Dariusz Wydra. 2020. "BARD1 and Breast Cancer: The Possibility of Creating Screening Tests and New Preventive and Therapeutic Pathways for Predisposed Women" Genes 11, no. 11: 1251. https://0-doi-org.brum.beds.ac.uk/10.3390/genes11111251