Mitochondrial Genomes Yield Insights into the Basal Lineages of Ichneumonid Wasps (Hymenoptera: Ichneumonidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Species Identification and DNA Extraction
2.2. Next-Generation Sequencing and Assembly
2.3. Mitochondrial Genes Annotation
2.4. Comparative Analysis of the Mitochondrial Genomes from Ichneumonidae
2.5. Phylogenetic Analysis
3. Results and Discussion
3.1. General Features of Mitochondrial Genomes
3.2. Base Composition, Codon Usage and Evolutionary Rate
3.3. Gene Rearrangement
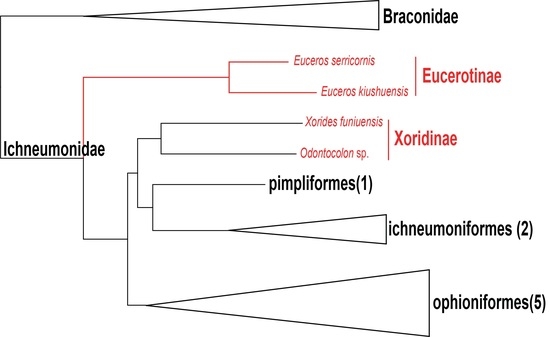
3.4. Phylogenetic Relationships within Ichneumonidae Based on Mitochondrial Genomes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, S.; Shi, M.; Sharkey, M.J.; van Achterberg, C.; Chen, X. Comparative mitogenomics of Braconidae (Insecta: Hymenoptera) and the phylogenetic utility of mitochondrial genomes with special reference to Holometabolous insects. BMC Genom. 2010, 11, 1471–2164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, R.S.; Krogmann, L.; Mayer, C.; Rust, J.; Misof, B.; Niehuis, O.; Peters, R.S.; Krogmann, L.; Mayer, C.; Donath, A.; et al. Evolutionary History of the Hymenoptera Report Evolutionary History of the Hymenoptera. Curr. Biol. 2017, 27, 1013–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broad, G.R.; Shaw, M.R.; Fitton, M.G. Ichneumonid wasps (Hymenoptera: Ichneumonidae): Their classification and biology. In Handbooks for the Identification of British Insects; Field Studies Council and Royal Entomological Society: Telford to St Albans, UK, 2018; Volume 7, pp. 1–418. ISBN 9781910159026. [Google Scholar]
- Klopfstein, S.; Santos, B.F.; Shaw, M.R.; Alvarado, M.; Bennett, A.M.R.; Dal Pos, D.; Giannotta, M.; Herrera Florez, A.F.; Karlsson, D.; Khalaim, A.I.; et al. Darwin wasps: A new name heralds renewed efforts to unravel the evolutionary history of Ichneumonidae. Entomol. Commun. 2019, 1, ec01006. [Google Scholar] [CrossRef]
- Quicke, D.L.J. The Braconid and Ichneumonid Parasitoid Wasps: Biology, Systematics, Evolution and Ecology; John Wiley & Sons, Ltd.: Chichester, UK, 2015; ISBN 9781118907085. [Google Scholar]
- Klopfstein, S.; Langille, B.; Spasojevic, T.; Broad, G.R.; Cooper, S.J.B.; Austin, A.D.; Niehuis, O. Hybrid capture data unravel a rapid radiation of pimpliform parasitoid wasps (Hymenoptera: Ichneumonidae: Pimpliformes). Syst. Entomol. 2019, 44, 361–383. [Google Scholar] [CrossRef]
- Sharanowski, B.J.; Ridenbaugh, R.D.; Piekarski, P.K.; Broad, G.R.; Burke, G.R.; Deans, A.R.; Lemmon, A.R.; Moriarty Lemmon, E.C.; Diehl, G.J.; Whitfield, J.B.; et al. Phylogenomics of Ichneumonoidea (Hymenoptera) and implications for evolution of mode of parasitism and viral endogenization. Mol. Phylogenet. Evol. 2021, 156, 107023. [Google Scholar] [CrossRef]
- Bennett, A.M.R.; Cardinal, S.; Gauld, I.D.; Wahl, D.B. Phylogeny of the subfamilies of Ichneumonidae (Hymenoptera). J. Hymenopt. Res. 2019, 156, 1–156. [Google Scholar] [CrossRef]
- Quicke, D.L.J.; Laurenne, N.M.; Fitton, M.G.; Broad, G.R. A thousand and one wasps: A 28S rDNA and morphological phylogeny of the Ichneumonidae (Insecta: Hymenoptera) with an investigation into alignment parameter space and elision. J. Nat. Hist. 2009, 43, 1305–1421. [Google Scholar] [CrossRef]
- Santos, B.F.; Wahl, D.B.; Rousse, P.; Bennett, A.M.R.; Kula, R.; Brady, S.G. Phylogenomics of Ichneumoninae (Hymenoptera, Ichneumonidae) reveals pervasive morphological convergence and the shortcomings of previous classifications. Syst. Entomol. 2021, 46, 704–724. [Google Scholar] [CrossRef]
- Santos, B.F. Phylogeny and reclassification of Cryptini (Hymenoptera, Ichneumonidae, Cryptinae), with implications for ichneumonid higher-level classification. Syst. Entomol. 2017, 650–676. [Google Scholar] [CrossRef]
- Quicke, D.L.J.; Lopez-Vaamonde, C.; Belshaw, R. The basal Ichneumonidae (Insecta, Hymenoptera): 28S D2 rDNA considerations of the Brachycyrtinae, Labeninae, Paxylommatinae and Xoridinae. Zool. Scr. 1999, 28, 203–210. [Google Scholar] [CrossRef]
- Cameron, S.L. Insect mitochondrial genomics: Implications for evolution and phylogeny. Annu. Rev. Entomol. 2014, 59, 95–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowton, M.; Castro, L.R.; Austin, A.D. Mitochondrial gene rearrangements as phylogenetic characters in the invertebrates: The examination of genome “morphology”. Invertebr. Syst. 2002, 16, 345–356. [Google Scholar] [CrossRef]
- Tang, P.; Zhu, J.C.; Zheng, B.Y.; Wei, S.J.; Sharkey, M.; Chen, X.X.; Vogler, A.P. Mitochondrial phylogenomics of the Hymenoptera. Mol. Phylogenet. Evol. 2018, 131, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.Y.; Cao, L.J.; Tang, P.; van Achterberg, K.; Ho, A.A.; Chen, H.Y.; Chen, X.X.; Wei, S.J. Molecular phylogenetics and evolution gene arrangement and sequence of mitochondrial genomes yield insights into the phylogeny and evolution of bees and sphecid wasps (Hymenoptera: Apoidea). Mol. Phylogenet. Evol. 2018, 124, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, H.; Song, F.; Zhao, Y.; Wilson, J.J.; Cai, W. Higher-level phylogeny and evolutionary history of Pentatomomorpha (Hemiptera: Heteroptera) inferred from mitochondrial genome sequences. Syst. Entomol. 2019, 44, 810–819. [Google Scholar] [CrossRef]
- Nie, R.E.; Andújar, C.; Gómez-Rodríguez, C.; Bai, M.; Xue, H.J.; Tang, M.; Yang, C.T.; Tang, P.; Yang, X.K.; Vogler, A.P. The phylogeny of leaf beetles (Chrysomelidae) inferred from mitochondrial genomes. Syst. Entomol. 2020, 45, 188–204. [Google Scholar] [CrossRef]
- Dowton, M.; Cameron, S.L.; Dowavic, J.I.; Austin, A.D.; Whiting, M.F. Characterization of 67 mitochondrial tRNA gene rearrangements in the Hymenoptera suggests that mitochondrial tRNA gene position is selectively neutral. Mol. Biol. Evol. 2009, 26, 1607–1617. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, D.C.S.G.; Raychoudhury, R.; Lavrov, D.V.; Werren, J.H. Rapidly evolving mitochondrial genome and directional selection in mitochondrial genes in the parasitic wasp Nasonia (Hymenoptera: Pteromalidae). Mol. Biol. Evol. 2008, 25, 2167–2180. [Google Scholar] [CrossRef] [Green Version]
- Sprevak, D.; Meegan, D. The Evolution of strand-specific compositional bias. A case study in the Hymenopteran mitochondrial 16s rRNA gene. Technometrics 1979, 21, 395. [Google Scholar] [CrossRef]
- Besnard, G.; Bertrand, J.A.M.; Delahaie, B.; Bourgeois, Y.X.C.; Lhuillier, E.; Thébaud, C. Valuing museum specimens: High-throughput DNA sequencing on historical collections of New Guinea crowned pigeons (Goura). Biol. J. Linn. Soc. 2016, 117, 71–82. [Google Scholar] [CrossRef] [Green Version]
- Andersen, J.C.; Mills, N.J. DNA extraction from museum specimens of parasitic Hymenoptera. PLoS ONE 2012, 7, e45549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alex, C.P.; Timmermans, M.J.T.N.; Gimmel, M.L.; Sujatha Narayanan, K.; Cockerill, T.D.; Chey, V.K.; Vogler, A.P. Soup to Tree: The phylogeny of beetles inferred by mitochondrial metagenomics of a Bornean rainforest sample. Mol. Biol. Evol. 2015, 32, 2302–2316. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, P.P.; Lowe, T.M. tRNAscan-SE: Searching for tRNA genes in genomic sequences. In Gene Prediction; Kollmar, M., Ed.; University of Hertfordshire: Hatfield, UK, 1962; pp. 1–14. ISBN 9781493991723. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Ma, Y.; Zheng, B.; Zhu, J.; Van Achterberg, C.; Tang, P.; Chen, X. The first two mitochondrial genomes of wood wasps (Hymenoptera: Symphyta): Novel gene rearrangements and higher-level phylogeny of the basal hymenopterans. Int. J. Biol. Macromol. 2019, 123, 1189–1196. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. De DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Nakamura, T.; Yamada, K.D.; Tomii, K.; Katoh, K. Parallelization of MAFFT for large-scale multiple sequence alignments. Bioinformatics 2018, 34, 2490–2492. [Google Scholar] [CrossRef] [Green Version]
- Golubchik, T.; Wise, M.J.; Easteal, S.; Jermiin, L.S. Mind the gaps: Evidence of bias in estimates of multiple sequence alignments. Mol. Biol. Evol. 2006, 24, 2433–2442. [Google Scholar] [CrossRef] [Green Version]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; Von Haeseler, A.; Lanfear, R.; Teeling, E. IQ-TREE2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [Green Version]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2018, 34, 772–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Wei, S.; Shi, M.; Chen, X.; Li, Q.; Wei, S.; Shi, M.; Chen, X. The mitochondrial genome of Diadromus collaris (Hymenoptera: Ichneumonidae). Mitochondrial DNA 2015, 26, 303–304. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Shi, M.; He, J.; Sharkey, M.; Chen, X. The complete mitochondrial genome of Diadegma semiclausum (Hymenoptera: Ichneumonidae) indicates extensive independent evolutionary events. Genome 2009, 52, 308–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jukes, T.H.; Cantor, C.R. Evolution of protein molecules. In Mammalian Protein Metabolism; Munro, H.N., Ed.; Academic Press: New York, NY, USA, 1969; pp. 21–132. [Google Scholar]




| Subfamily | Species | Accession Number | Reference |
|---|---|---|---|
| Ichneumoninae | Amblyjoppa sp. | MG923483 | Tang et al. [15] |
| Ichneumoninae | Diadromus collaris | JX131613 | Li et al. [35] |
| Ophioninae | Enicospilus sp. | FJ478177 | Dowton et al. [19] |
| Campopleginae | Venturia canescens | FJ478176 | Dowton et al. [19] |
| Campopleginae | Hyposoter sp. | MG923499 | Tang et al. [15] |
| Campopleginae | Diadegma semiclausum | EU871947 | Wei et al. [36] |
| Pimplinae | Pimpla luctuosa | MG923506 | Tang et al. [15] |
| Metopiinae | Hypsicera sp. | MG923500 | Tang et al. [15] |
| Xoridinae | Odontocolon sp. | MT252850 | This study |
| Xoridinae | Xorides funiuensis | MT252851 | This study |
| Eucerotinae | Euceros kiushuensis | MT252852 | This study |
| Eucerotinae | Euceros serricornis | MT252853 | This study |
| Euphorinae (outgroup) | Zele chlorophthalmus | MG822749 | Direct Submission |
| Microgastrinae (outgroup) | Cotesia vestalis | FJ154897 | Wei et al. [1] |
| Species | Sequences Length (bp) | Protein-Coding Genes (PCGs) | |||
|---|---|---|---|---|---|
| (A + T) % | AT-Skew | GC-Skew | Length (bp) | ||
| Amblyjoppa sp. | 17,110 | 80.07 | −0.1113 | −0.0687 | 11,097 |
| Diadromus collaris | 14,621 | 82.86 | −0.1147 | −0.0421 | 11,088 |
| Enicospilus sp. | 15,300 | 84.01 | −0.1118 | 0.0330 | 11,175 |
| Venturia canescens | 13,635 | 84.01 | −0.1277 | −0.0050 | 10,008 |
| Hyposoter sp. | 18,893 | 83.45 | −0.1200 | −0.0227 | 11,202 |
| Diadegma semiclausum | 18,728 | 83.76 | −0.1205 | −0.0039 | 11,166 |
| Pimpla luctuosa | 16,926 | 81.11 | −0.1210 | −0.0258 | 11,088 |
| Hypsicera sp. | 17,017 | 78.26 | −0.1431 | −0.0412 | 11,175 |
| Odontocolon sp. | 13,321 | 78.60 | −0.1009 | −0.0521 | 11,199 |
| Xorides funiuensis | 14,939 | 82.35 | −0.1180 | −0.0214 | 11,130 |
| Euceros kiushuensis | 16,331 | 83.99 | −0.1627 | 0.0782 | 11,097 |
| Euceros serricornis | 15,787 | 83.53 | −0.1581 | 0.0537 | 11,088 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, B.; Han, Y.; Yuan, R.; Liu, J.; Tang, P.; van Achterberg, C.; Chen, X. Mitochondrial Genomes Yield Insights into the Basal Lineages of Ichneumonid Wasps (Hymenoptera: Ichneumonidae). Genes 2022, 13, 218. https://0-doi-org.brum.beds.ac.uk/10.3390/genes13020218
Zheng B, Han Y, Yuan R, Liu J, Tang P, van Achterberg C, Chen X. Mitochondrial Genomes Yield Insights into the Basal Lineages of Ichneumonid Wasps (Hymenoptera: Ichneumonidae). Genes. 2022; 13(2):218. https://0-doi-org.brum.beds.ac.uk/10.3390/genes13020218
Chicago/Turabian StyleZheng, Boying, Yuanyuan Han, Ruizhong Yuan, Jingxian Liu, Pu Tang, Cornelis van Achterberg, and Xuexin Chen. 2022. "Mitochondrial Genomes Yield Insights into the Basal Lineages of Ichneumonid Wasps (Hymenoptera: Ichneumonidae)" Genes 13, no. 2: 218. https://0-doi-org.brum.beds.ac.uk/10.3390/genes13020218