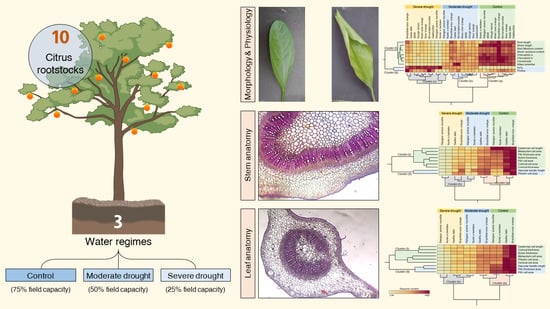
Effect of Three Water Regimes on the Physiological and Anatomical Structure of Stem and Leaves of Different Citrus Rootstocks with Distinct Degrees of Tolerance to Drought Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials, Experimental Site, and Growth Conditions
2.2. Water Regimes and Treatments
2.3. Morphological and Biomass Measurements
2.4. Biochemical and Stress-Associated Biomarker Measurements
2.4.1. Leaf Photosynthetic Pigments Contents
2.4.2. Determination of Proline
2.4.3. Determination of Hydrogen Peroxide (H2O2)
2.5. Stem and Leaf Anatomical Evaluation
2.5.1. Plant Material and Experiment
2.5.2. Preservation, Sectioning, Staining, and Mounting
2.5.3. Anatomical Traits
2.6. Statistical Analysis
3. Results
3.1. Drought Negatively Affects the Roots and Shoots Length
3.2. Drought Stress Disrupts the Water Relations of Citrus Rootstocks
3.3. Water Deficiency Interrupts the Photosynthetic Pigments of Citrus Rootstocks
3.4. Drought Stress Induced the Accumulation of Stress-Associated Biomarkers in Citrus Rootstocks
3.5. Principal Component Analysis (PCA) and Two-Way Hierarchical Cluster Analysis (HCA) Revealed the Differences among Water Treatments and Citrus Rootstocks
3.6. Water Deficiency Alters the Anatomical Structure of Citrus Rootstocks
3.6.1. Effect of Drought Stress on Stem Anatomy of Citrus Rootstocks
3.6.2. PCA and Two-Way HCA Divulged the Variations in Stem Anatomy of Different Citrus Rootstocks
3.6.3. Effect of Drought Stress on Leaf Tissue Structure of Citrus Rootstocks
3.6.4. PCA and Two-Way HCA Revealed the Differences in Leaf Tissue Structure of Different Citrus Rootstocks
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehdi, M.; Ashfaq, M.; Hassan, S.; Abid, M. Effect of marketing channel choice on the profitability of citrus farmers: Evidence form Punjab-Pakistan. Pak. J. Agric. Sci. 2019, 56, 1003–1011. [Google Scholar]
- Talat, H.; Shafqat, W.; Qureshi, M.A.; Sharif, N.; Raza, M.K.; Din, S.; Ikram, S.; Jaskani, M.J. Effect of gibberellic acid on fruit quality of Kinnow mandarin. J. Glob. Innov. Agric. Soc. Sci. 2020, 8, 59–63. [Google Scholar] [CrossRef]
- Silva, S.F.; Miranda, M.T.; Costa, V.E.; Machado, E.C.; Ribeiro, R.V. Sink strength of citrus rootstocks under water deficit. Tree Physiol. 2021, 41, 1372–1383. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.A.; Jaskani, M.J.; Khan, A.S.; Ahmad, R. Influence of endogenous plant hormones on physiological and growth attributes of Kinnow mandarin grafted on nine rootstocks. J. Plant Growth Regul. 2021, 1–11. [Google Scholar] [CrossRef]
- Qureshi, M.A.; Jaskani, M.J.; Khan, A.S.; Haider, M.S.; Shafqat, W.; Asif, M.; Mehmood, A. Influence of different rootstocks on physico-chemical quality attributes of Kinnow mandarin. Pak. J. Agric. Sci. 2021, 58, 929–935. [Google Scholar]
- Khan, K.; Ikram, S.; Ashfaq, M.; Jaskani, M.J.; Shafqat, W. Citrus rootstock characterization against citrus canker and evaluation of antibiotics effect against Xanthomonas axonopodis pv. Citri. J. Innov. Agric. 2021, 8, 1–8. [Google Scholar]
- Shafqat, W.; Tahir, T.; Khurshid, T.; Ur-Rahman, H.; Saqib, M.; Jaskani, M.J. Effect of rootstock types on leaf nutrient composition in three commercial citrus scion cultivars of Pakistan under the ASLP Citrus Project. In Proceedings of the XXIX International Horticultural Congress on Horticulture: Sustaining Lives, Livelihoods and Landscapes (IHC2014): 1128, Brisbane, Australia, 25 November 2016. [Google Scholar]
- Bowman, K.D.; Joubert, J. Citrus rootstocks. In The Genus Citrus; Woodhead Publishing Books—Elsevier: Sawston, UK, 2020; pp. 105–127. [Google Scholar]
- Morianou, G.; Ziogas, V.; Kourgialas, N.N.; Karatzas, G.P. Effect of irrigation practices upon yield and fruit quality of four grapefruit (Citrus paradisi Mac.) cultivars. Water Supply 2021, 21, 2735–2747. [Google Scholar] [CrossRef]
- Koshita, Y.; Takahara, T. Effect of water stress on flower-bud formation and plant hormone content of satsuma mandarin (Citrus unshiu Marc.). Sci. Hortic. 2004, 99, 301–307. [Google Scholar] [CrossRef]
- Syvertsen, J.; Hanlon, E.A. Citrus tree stresses: Effects on growth and yield. EDIS 2008, 2008, 1–6. [Google Scholar]
- Zaman, L.; Shafqat, W.; Qureshi, A.; Sharif, N.; Raza, K.; ud Din, S.; Ikram, S.; Jaskani, M.J. Effect of foliar spray of zinc sulphate and calcium carbonate on fruit quality of Kinnow mandarin (Citrus reticulata Blanco). J. Glob. Innov. Agric. Soc. Sci. 2019, 7, 157–161. [Google Scholar] [CrossRef]
- Bray, E.A.; Bailey-Serres, J.; Weretilnyk, E. Responses to abiotic stress. In Biochemistry & Molecular Biology of Plants; Gruissem, W., Jones, R., Eds.; American Society of Plant Physiologists: Rockville, MD, USA, 2000; pp. 1158–1203. [Google Scholar]
- Bates, B.; Kundzewicz, Z.; Wu, S. Climate Change and Water; Intergovernmental Panel on Climate Change Secretariat: Geneva, Switzerland, 2008; ISBN 9291691232. [Google Scholar]
- Ramírez, D.A.; Rolando, J.L.; Yactayo, W.; Monneveux, P.; Mares, V.; Quiroz, R. Improving potato drought tolerance through the induction of long-term water stress memory. Plant Sci. 2015, 238, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Zandalinas, S.I.; Rivero, R.M.; Martínez, V.; Gómez-Cadenas, A.; Arbona, V. Tolerance of citrus plants to the combination of high temperatures and drought is associated to the increase in transpiration modulated by a reduction in abscisic acid levels. BMC Plant Biol. 2016, 16, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnabás, B.; Jäger, K.; Fehér, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Müller, F.; Rieu, I.; Winter, P. Epigenetic events in plant male germ cell heat stress responses. Plant Reprod. 2016, 29, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, F. Drought stress memory and drought stress tolerance in plants: Biochemical and molecular basis. In Drought Stress Tolerance in Plants; Springer: New York, NY, USA, 2016; Volume 1, pp. 17–44. [Google Scholar]
- Kinoshita, T.; Seki, M. Epigenetic memory for stress response and adaptation in plants. Plant Cell Physiol. 2014, 55, 1859–1863. [Google Scholar] [CrossRef]
- Shafqat, W.; Jaskani, M.; Maqbool, R.; Sattar Khan, A. Evaluation of citrus rootstocks against drought, heat and their combined stress based on growth and photosynthetic pigments fingerprinting of Jamun (Syzygium cumini) genetic resources of Punjab view project. Int. J. Agri. Biol. 2019, 22, 1001–1009. [Google Scholar]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [Green Version]
- Xiong, B.; Wang, Y.; Zhang, Y.; Ma, M.; Gao, Y.; Zhou, Z.; Wang, B.; Wang, T.; Lv, X.; Wang, X. Alleviation of drought stress and the physiological mechanisms in Citrus cultivar (Huangguogan) treated with methyl jasmonate. Biosci. Biotechnol. Biochem. 2020, 84, 1958–1965. [Google Scholar] [CrossRef]
- Liu, S.; Yang, R. Regulations of reactive oxygen species in plants abiotic stress: An integrated overview. In Plant Life under Changing Environment: Responses and Management; Tripathi, D.K., Singh, V.P., Chauhan, D.K., Sharma, S., Prasad, S.M., Dubey, N.K., Ramawat, N., Eds.; Academic Press-Elsevier: Cambridge, MA, USA, 2020; pp. 323–353. [Google Scholar]
- Noctor, G.; Veljovic-Jovanovic, S.; Driscoll, S.; Novitskaya, L.; Foyer, C.H. Drought and oxidative load in the leaves of C3 plants: A predominant role for photorespiration? Ann. Bot. 2002, 89, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.Y.; Yu, H.Y.; Yang, M.M.; Kong, D.S.; Zhang, Y.J. Effect of drought stress on lipid peroxidation, osmotic adjustment and antioxidant enzyme activity of leaves and roots of Lycium ruthenicum Murr. seedling. Russ. J. Plant Physiol. 2018, 65, 244–250. [Google Scholar] [CrossRef]
- Baker, N.R.; Rosenqvist, E. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Matos Nunes, J.; Bertodo, L.O.O.; Da Rosa, L.M.G.; Von Poser, G.L.; Rech, S.B. Stress induction of valuable secondary metabolites in Hypericum polyanthemum acclimatized plants. S. Afr. J. Bot. 2014, 94, 182–189. [Google Scholar] [CrossRef] [Green Version]
- Zaefyzadeh, M.; Quliyev, R.A.; Babayeva, S.M.; Abbasov, M.A. The effect of the interaction between genotypes and drought stress on the superoxide dismutase and chlorophyll content in durum wheat landraces. Turk. J. Biol. 2009, 33, 1–7. [Google Scholar]
- Osmolovskaya, N.; Shumilina, J.; Kim, A.; Didio, A.; Grishina, T.; Bilova, T.; Keltsieva, O.A.; Zhukov, V.; Tikhonovich, I.; Tarakhovskaya, E. Methodology of drought stress research: Experimental setup and physiological characterization. Int. J. Mol. Sci. 2018, 19, 4089. [Google Scholar] [CrossRef] [Green Version]
- Nyachiro, J.M.; Briggs, K.G.; Hoddinott, J.; Johnson-Flanagan, A.M. Chlorophyll content, chlorophyll fluorescence and water deficit in spring wheat. Cereal Res. Commun. 2001, 29, 135–142. [Google Scholar] [CrossRef]
- Kyparissis, A.; Grammatikopoulos, G.; Manetas, Y. Leaf morphological and physiological adjustments to the spectrally selective shade imposed by anthocyanins in Prunus cerasifera. Tree Physiol. 2007, 27, 849–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline mechanisms of stress survival. Antioxid. Redox Signal. 2013, 19, 998–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef]
- Matsuda, K.; Rayan, A. Anatomy: A key factor regulating plant tissue response to water stress. In Environment Injury to Plants; Kafternan, F., Ed.; Academic Press-Elsevier: Cambridge, MA, USA, 1990; p. 290. [Google Scholar]
- Olmos, E.; Sánchez-Blanco, M.J.; Ferrández, T.; Alarcon, J.J. Subcellular effects of drought stress in Rosmarinus officinalis. Plant Biol. 2007, 9, 77–84. [Google Scholar] [CrossRef]
- Tombesi, S.; Johnson, R.S.; Day, K.R.; DeJong, T.M. Relationships between xylem vessel characteristics, calculated axial hydraulic conductance and size-controlling capacity of peach rootstocks. Ann. Bot. 2009, 105, 327–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlquist, S. Comparative Wood Anatomy: Systematic, Ecological, and Evolutionary Aspects of Dicotyledon Wood; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; ISBN 3662217147. [Google Scholar]
- Pitman, W.D.; Holt, E.C.; Conrad, B.E.; Bashaw, E.C. Histological differences in moisture-stressed and nonstressed Kleingrass forage 1. Crop. Sci. 1983, 23, 793–795. [Google Scholar] [CrossRef]
- Guerfel, M.; Baccouri, O.; Boujnah, D.; Chaïbi, W.; Zarrouk, M. Impacts of water stress on gas exchange, water relations, chlorophyll content and leaf structure in the two main Tunisian olive (Olea europaea L.) cultivars. Sci. Hortic. 2009, 119, 257–263. [Google Scholar] [CrossRef]
- Child, R.D.; Summers, J.E.; Babij, J.; Farrent, J.W.; Bruce, D.M. Increased resistance to pod shatter is associated with changes in the vascular structure in pods of a resynthesized Brassica napus line. J. Exp. Bot. 2003, 54, 1919–1930. [Google Scholar] [CrossRef]
- Lo Gullo, M.A.; Salleo, S.; Piaceri, E.C.; Rosso, R. Relations between vulnerability to xylem embolism and xylem conduit dimensions in young trees of Quercus corris. Plant Cell Environ. 1995, 18, 661–669. [Google Scholar] [CrossRef]
- Trifilo, P.; Lo Gullo, M.A.; Nardini, A.; Pernice, F.; Salleo, S. Rootstock effects on xylem conduit dimensions and vulnerability to cavitation of Olea europaea L. Trees 2007, 21, 549–556. [Google Scholar] [CrossRef]
- Meland, M.; Moe, M.E.; Frøynes, O. Differences in growth and development of functional xylem of grafted and budded sweet cherry trees. In Proceedings of the VIII International Symposium on Canopy, Rootstocks and Environmental Physiology in Orchard Systems, Budapest, Hungary, 13–18 June 2004; pp. 311–316. [Google Scholar]
- Zach, A.; Schuldt, B.; Brix, S.; Horna, V.; Culmsee, H.; Leuschner, C. Vessel diameter and xylem hydraulic conductivity increase with tree height in tropical rainforest trees in Sulawesi, Indonesia. Flora-Morphol. Distrib. Funct. Ecol. Plants 2010, 205, 506–512. [Google Scholar] [CrossRef]
- Tyree, M.T.; Ewers, F.W. The hydraulic architecture of trees and other woody plants. New Phytol. 1991, 119, 345–360. [Google Scholar] [CrossRef]
- Şahin-Çevik, M.; Çevik, B.; Topkaya-Kütük, B.; Yazıcı, K. Identification of drought-induced genes from the leaves of Rangpur lime (Citrus limon (L) Osbeck). J. Hortic. Sci. Biotechnol. 2017, 92, 636–645. [Google Scholar] [CrossRef]
- Shafqat, W.; Jaskani, M.J.; Maqbool, R.; Chattha, W.S.; Ali, Z.; Naqvi, S.A.; Haider, M.S.; Khan, I.A.; Vincent, C.I. Heat shock protein and aquaporin expression enhance water conserving behavior of citrus under water deficits and high temperature conditions. Environ. Exp. Bot. 2021, 181, 104270. [Google Scholar] [CrossRef]
- Ábrahám, E.; Hourton-Cabassa, C.; Erdei, L.; Szabados, L. Methods for determination of proline in plants. In Plant Stress Tolerance; Humana Press-Springer: Totowa, NJ, USA, 2010; pp. 317–331. [Google Scholar]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Hussain, S.; Khalid, M.F.; Saqib, M.; Ahmad, S.; Zafar, W.; Rao, M.J.; Morillon, R.; Anjum, M.A. Drought tolerance in citrus rootstocks is associated with better antioxidant defense mechanism. Acta Physiol. Plant 2018, 40, 135. [Google Scholar] [CrossRef]
- Santana-Vieira, D.D.S.; Freschi, L.; da Hora Almeida, L.A.; de Moraes, D.H.S.; Neves, D.M.; Dos Santos, L.M.; Bertolde, F.Z.; dos Santos Soares Filho, W.; Coelho Filho, M.A.; da Silva Gesteira, A. Survival strategies of citrus rootstocks subjected to drought. Sci. Rep. 2016, 6, 38775. [Google Scholar] [CrossRef]
- Aroca, R. Plant responses to drought stress. In From Morphological to Molecular Features; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Zhang, J.; Kirkham, M.B. Water status of drought-resistant and drought-sensitive sorghum treated with ethephon. J. Plant Growth Regul. 1990, 9, 189–194. [Google Scholar] [CrossRef]
- Savé, R.; Biel, C.; Domingo, R.; Ruiz-Sánchez, M.C.; Torrecillas, A. Some physiological and morphological characteristics of citrus plants for drought resistance. Plant Sci. 1995, 110, 167–172. [Google Scholar] [CrossRef]
- Homayoun, H.; Daliri, M.S.; Mehrabi, P. Effect of drought stress on leaf chlorophyll in corn cultivars (Zea mays). Middle East. J. Sci. Res. 2011, 9, 418–420. [Google Scholar]
- Mafakheri, A.; Siosemardeh, A.F.; Bahramnejad, B.; Struik, P.C.; Sohrabi, Y. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust. J. Crop. Sci. 2010, 4, 580–585. [Google Scholar]
- Nawazish, S.; Hameed, M.; Naurin, S. Leaf anatomical adaptations of Cenchrus ciliaris L. from the Salt Range, Pakistan against drought stress. Pak. J. Bot. 2006, 38, 1723–1730. [Google Scholar]
- Molassiotis, A.; Job, D.; Ziogas, V.; Tanou, G. Citrus plants: A model system for unlocking the secrets of NO and ROS-inspired priming against salinity and drought. Front. Plant Sci. 2016, 7, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [Green Version]
- Chartzoulakis, K.; Patakas, A.; Bosabalidis, A.M. Changes in water relations, photosynthesis and leaf anatomy induced by intermittent drought in two olive cultivars. Environ. Exp. Bot. 1999, 42, 113–120. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, J.; Zhang, H.; Xu, H.; Wang, H.; Xie, M. Gene expression profiling in response to drought stress in citrus leaves by cDNA-AFLP. Acta Hortic. Sin. 2011, 38, 417–424. [Google Scholar]
- Wu, Q.-S.; Srivastava, A.K.; Zou, Y.-N. AMF-induced tolerance to drought stress in citrus: A review. Sci. Hortic. 2013, 164, 77–87. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Martel, A.B.; Dixon, S.L. Environmental factors influence plant vascular system and water regulation. Plants 2019, 8, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balfagón, D.; Terán, F.; de Oliveira, T.; Santa-Catarina, C.; Gómez-Cadenas, A. Citrus rootstocks modify scion antioxidant system under drought and heat stress combination. Plant Cell Rep. 2021, 1–10. [Google Scholar] [CrossRef]
- Mansoor, U.; Fatima, S.; Hameed, M.; Naseer, M.; Ahmad, M.S.A.; Ashraf, M.; Ahmad, F.; Waseem, M. Structural modifications for drought tolerance in stem and leaves of Cenchrus ciliaris L. ecotypes from the Cholistan Desert. Flora 2019, 261, 151485. [Google Scholar] [CrossRef]
- Crous, C.J.; Greyling, I.; Wingfield, M.J. Dissimilar stem and leaf hydraulic traits suggest varying drought tolerance among co-occurring Eucalyptus grandis × E. urophylla clones. South. For. J. For. Sci. 2018, 80, 175–184. [Google Scholar] [CrossRef]








| Rootstock | Botanical Name | Citrus Category | Leaf Shape | Parentage/Origin |
|---|---|---|---|---|
| Gabbuchini | Citrus aurantium L. | Sour orange hybrid | Unifoliate | C. aurantium ‘Bittersweet’ × C. sinensis |
| Gada dahi | Citrus maxima Burm. Merrill/ Citrus grandis L. Osbeck/ Citrus decumana L. | Pummelo hybrid | Unifoliate | Subcontinent (Indo-Pak), seed selection |
| Sour orange | Citrus aurantium L. | Sour orange | Unifoliate | Subcontinent (Indo-Pak) |
| Keen sour orange | Citrus aurantium L. | Sour orange | Unifoliate | Selection/root sprout |
| Brazilian sour orange | Citrus aurantium L. | Sour orange | Unifoliate | Open-pollinated seed selection |
| Rough lemon | Citrus jambhiri | Lemon | Unifoliate | Open-pollinated seed selection |
| Sunki × bentake, | Citrus spp. | Unknown | Trifoliate | Citrus sunki × bentake hybrid |
| X639 | Citroncirus spp. | Mandarin × Poncirus | Trifoliate | Cleopatra mandarin × Poncirus trifoliata hybrid |
| Kirrumakki nucellar | Citrus limonia Osbeck | Lime | Unifoliate | Unknow/Subcontinent (Indo-Pak) |
| Rangpur Poona nucellar | Citrus limonia Osbeck | Lime | Unifoliate | Unknow/Subcontinent (Indo-Pak) |
| Rootstock | Root Length (cm) | Shoot Length (cm) | Water Potential (Mpa) | Moisture Content | ||
|---|---|---|---|---|---|---|
| Root (%) | Shoot (%) | |||||
| Control | Gabbuchini | 25.33 ± 0.58 ab | 21.00 ± 1.00 b | −0.32 ± 0.02 a–e | 66.00 ± 1.00 cde | 73.92 ± 1.12 |
| Gada dahi | 24.33 ± 0.58 bc | 24.33 ± 0.58 a | −0.23 ± 0.02 a–d | 69.00 ± 1.00 b | 77.28 ± 1.12 b | |
| Sour orange | 24.33 ± 0.58 bc | 23.67 ± 0.58 a | −0.27 ± 0.06 a–e | 64.67 ± 0.58 e | 72.43 ± 0.65 e | |
| Keen sour orange | 25.33 ± 0.58 ab | 25.33 ± 0.58 a | −0.19 ± 0.02 abc | 67.33 ± 0.58 bcd | 75.41 ± 0.65 bcd | |
| Rough lemon | 26.00 ± 1.00 ab | 25.00 ± 1.00 a | −0.19 ± 0.03 abc | 64.67 ± 0.58 e | 72.43 ± 0.65 e | |
| Brazilian sour orange | 27.67 ± 0.58 a | 25.00 ± 1.00 a | −0.23 ± 0.02 a–d | 75.67 ± 0.58 a | 84.75 ± 0.65 a | |
| Sunki × bentake | 12.33 ± 0.58 jkl | 24.67 ± 0.58 a | −0.28 ± 0.05 a–e | 65.33 ± 0.58 de | 73.17 ± 0.65 de | |
| X639 | 24.33 ± 0.58 bc | 24.33 ± 0.58 a | −0.26 ± 0.06 a–e | 67.00 ± 1.00 b–e | 75.04 ± 1.12 b–e | |
| Kirrumakki nucellar | 24.00 ± 1.00 bc | 24.00 ± 1.00 a | −0.23 ± 0.02 a–d | 68.00 ± 1.00 bc | 76.16 ± 1.12 bc | |
| Rangpur poona nucellar | 24.67 ± 0.58 bc | 24.67 ± 0.58 a | −0.23 ± 0.02 a–d | 66.00 ± 1.00 cde | 73.92 ± 1.12 cde | |
| Moderate drought | Gabbuchini | 15.67 ± 0.58 ghi | 12.00 ± 1.00 ghi | −0.60 ± 0.05 a–f | 55.67 ± 0.58 fg | 62.35 ± 0.65 fg |
| Gada dahi | 22.67 ± 0.58 cd | 18.33 ± 0.58 c | −0.15 ± 0.58 ab | 64.67 ± 0.58 e | 72.43 ± 0.65 e | |
| Sour orange | 19.00 ± 1.00 ef | 15.67 ± 0.58 de | −0.61 ± 0.06 a–f | 54.00 ± 1.00 g | 60.48 ± 1.12 g | |
| Keen sour orange | 21.00 ± 1.00 de | 18.33 ± 0.58 c | −0.62 ± 0.14 a–f | 58.00 ± 1.00 f | 64.96 ± 1.12 f | |
| Rough lemon | 17.33 ± 0.58 fg | 14.33 ± 0.58 efg | −0.68 ± 0.03 b–f | 54.33 ± 0.58 g | 60.85 ± 0.65 g | |
| Brazilian sour orange | 25.67 ± 0.58 ab | 19.00 ± 1.00 bc | −0.70 ± 0.03 c–f | 65.00 ± 1.00 de | 72.80 ± 1.12 de | |
| Sunki × bentake | 10.33 ± 0.58 lm | 14.67 ± 0.58 def | −0.66 ± 0.04 b–f | 50.33 ± 0.58 h | 56.37 ± 0.65 h | |
| X639 | 16.33 ± 1.53 gh | 13.00 ± 1.00 fgh | −0.74 ± 0.06 def | 54.33 ± 0.58 g | 60.85 ± 0.65 g | |
| Kirrumakki nucellar | 15.33 ± 0.58 ghi | 12.00 ± 1.00 ghi | −0.11 ± 0.66 a | 54.00 ± 1.00 g | 60.48 ± 1.12 g | |
| Rangpur poona nucellar | 14.67 ± 0.58 hij | 11.00 ± 1.00 hij | −0.78 ± 0.03 efg | 48.67 ± 0.58 hi | 54.51 ± 0.65 hi | |
| Severe drought | Gabbuchini | 11.67 ± 1.15 klm | 08.33 ± 0.58 kl | −1.30 ± 0.06 gh | 46.33 ± 0.58 ij | 51.89 ± 0.65 ij |
| Gada dahi | 19.33 ± 0.58 ef | 14.67 ± 0.58 def | −1.14 ± 0.01 fgh | 54.67 ± 0.58 g | 61.23 ± 0.65 g | |
| Sour orange | 14.67 ± 0.58 hij | 11.67 ± 0.58 hi | −1.30 ± 0.05 gh | 46.67 ± 0.58 ij | 52.27 ± 0.65 ij | |
| Keen sour orange | 17.33 ± 0.58 fg | 13.00 ± 1.00 fgh | −1.39 ± 0.07 h | 50.00 ± 1.00 h | 56.00 ± 1.12 h | |
| Rough lemon | 13.67 ± 0.58 ijk | 10.33 ± 0.58 ijk | −1.39 ± 0.04 h | 45.00 ± 1.00 jk | 50.40 ± 1.12 jk | |
| Brazilian sour orange | 24.33 ± 0.58 bc | 17.00 ± 1.00 cd | −1.57 ± 0.03 h | 57.67 ± 1.53 f | 64.59 ± 1.71 f | |
| Sunki × bentake | 09.33 ± 0.58 lm | 06.00 ± 1.00 lm | −1.39 ± 0.03 h | 39.67 ± 0.58 l | 44.43 ± 0.65 l | |
| X639 | 14.00 ± 1.00 hijk | 08.33 ± 0.58 kl | −1.36 ± 0.06 h | 43.33 ± 0.58 k | 48.53 ± 0.65 k | |
| Kirrumakki nucellar | 13.33 ± 0.58 ijk | 08.67 ± 0.58 jk | −1.38 ± 0.03 h | 45.00 ± 1.00 jk | 50.40 ± 1.12 jk | |
| Rangpur poona nucellar | 10.33 ± 0.58 lm | 05.33 ± 0.58 m | −1.40 ± 0.03 h | 36.33 ± 0.58 m | 40.69 ± 0.65 m | |
| p-value | ||||||
| pDrought | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| pRootstock | <0.0001 | <0.0001 | =0.0018 | <0.0001 | <0.0001 | |
| pDrought × Rootstock | <0.0001 | <0.0001 | =0.0250 | <0.0001 | <0.0001 | |
| Rootstock | Chlorophyll a (mg g−1 FW) | Chlorophyll b (mg g−1 FW) | Carotenoids (mg g−1 FW) | H2O2 (µmol g−1 FW) | Proline (µmol g−1 FW) | |
|---|---|---|---|---|---|---|
| Control | Gabbuchini | 3.52 ± 0.03 b | 1.43 ± 0.06 abc | 1.05 ± 0.04 ab | 43.00 ± 2.65 d | 0.33 ± 0.03 c–h |
| Gada dahi | 3.54 ± 0.01 ab | 1.47 ± 0.06 ab | 1.13 ± 0.04 a | 44.33 ± 5.03 d | 0.35 ± 0.01 c–g | |
| Sour orange | 3.53 ± 0.02 b | 1.53 ± 0.06 a | 1.18 ± 0.04 a | 42.33 ± 1.15 d | 0.38 ± 0.02 c–f | |
| Keen sour orange | 3.57 ± 0.05 ab | 1.50 ± 0.10 a | 1.15 ± 0.08 a | 46.00 ± 2.00 d | 0.29 ± 0.00 fgh | |
| Rough lemon | 3.60 ± 0.08 ab | 1.50 ± 0.10 a | 1.15 ± 0.08 a | 45.33 ± 2.52 d | 0.32 ± 0.03 c–h | |
| Brazilian sour orange | 3.74 ± 0.12 a | 1.57 ± 0.06 a | 1.26 ± 0.12 a | 45.00 ± 2.65 d | 0.38 ± 0.02 c–f | |
| Sunki × bentake | 3.50 ± 0.05 b | 1.53 ± 0.06 a | 1.18 ± 0.04 a | 44.67 ± 0.58 d | 0.25 ± 0.01 ghi | |
| X639 | 3.51 ± 0.06 b | 1.57 ± 0.06 a | 1.21 ± 0.04 a | 39.67 ± 6.03 d | 0.26 ± 0.00 ghi | |
| Kirrumakki nucellar | 3.57 ± 0.08 ab | 1.57 ± 0.06 a | 1.26 ± 0.04 a | 42.67 ± 1.53 d | 0.41 ± 0.01 bcd | |
| Rangpur poona nucellar | 3.68 ± 0.04 ab | 1.53 ± 0.06 a | 1.18 ± 0.04 a | 44.67 ± 2.08 d | 0.17 ± 0.02 i–l | |
| Moderate drought | Gabbuchini | 2.95 ± 0.04 de | 1.23 ± 0.06 cde | 0.77 ± 0.08 c–f | 64.33 ± 2.08 c | 0.18 ± 0.03 i–l |
| Gada dahi | 3.10 ± 0.01 cde | 1.27 ± 0.06 bcd | 0.85 ± 0.05 bcd | 68.33 ± 3.06 bc | 0.29 ± 0.01 fgh | |
| Sour orange | 2.99 ± 0.04 cde | 1.03 ± 0.12 efg | 0.79 ± 0.09 cde | 64.67 ± 1.53 c | 0.26 ± 0.04 ghi | |
| Keen sour orange | 3.12 ± 0.02 cde | 1.23 ± 0.06 cde | 0.82 ± 0.12 cde | 62.67 ± 2.08 c | 0.23 ± 0.06 hij | |
| Rough lemon | 3.14 ± 0.03 cd | 0.93 ± 0.15 fgh | 0.72 ± 0.12 c–f | 62.00 ± 1.00 c | 0.25 ± 0.05 ghi | |
| Brazilian sour orange | 3.19 ± 0.04 c | 1.37 ± 0.06 abc | 0.87 ± 0.04 bcd | 66.00 ± 2.65 c | 0.31 ± 0.02 d–h | |
| Sunki × bentake | 2.66 ± 0.05 g | 0.80 ± 0.10 hi | 0.61 ± 0.05 e–h | 66.00 ± 1.73 c | 0.14 ± 0.05 jkl | |
| X639 | 3.11 ± 0.03 cde | 1.13 ± 0.06 def | 0.79 ± 0.09 cde | 60.67 ± 1.53 c | 0.25 ± 0.02 ghi | |
| Kirrumakki nucellar | 2.92 ± 0.03 ef | 1.13 ± 0.06 def | 0.90 ± 0.12 bc | 64.67 ± 1.53 c | 0.41 ± 0.00 bcd | |
| Rangpur poona nucellar | 2.54 ± 0.02 gh | 0.73 ± 0.06 h–k | 0.57 ± 0.07 f–i | 64.67 ± 2.52 c | 0.08 ± 0.06 l | |
| Severe drought | Gabbuchini | 2.20 ± 0.03 jk | 0.77 ± 0.06 hij | 0.44 ± 0.04 c–f | 83.00 ± 2.00 a | 0.41 ± 0.01 abc |
| Gada dahi | 2.45 ± 0.04 hi | 0.83 ± 0.06 ghi | 0.66 ± 0.01 d–g | 83.33 ± 2.08 a | 0.40 ± 0.00 cde | |
| Sour orange | 2.31 ± 0.03 ij | 0.63 ± 0.06 i–l | 0.41 ± 0.04 hij | 84.00 ± 2.65 a | 0.38 ± 0.06 c–f | |
| Keen sour orange | 2.24 ± 0.06 ijk | 0.63 ± 0.06 i–l | 0.49 ± 0.04 ghi | 82.33 ± 3.06 a | 0.34 ± 0.03 c–g | |
| Rough lemon | 2.71 ± 0.25 fg | 0.57 ± 0.06 jkl | 0.38 ± 0.08 ij | 84.00 ± 1.00 a | 0.29 ± 0.04 fgh | |
| Brazilian sour orange | 2.70 ± 0.06 g | 0.87 ± 0.06 gh | 0.69 ± 0.08 c–g | 77.00 ± 1.00 ab | 0.51 ± 0.06 a | |
| Sunki × bentake | 1.87 ± 0.04 l | 0.47 ± 0.06 l | 0.23 ± 0.02 jk | 85.67 ± 4.16 a | 0.18 ± 0.01 ijk | |
| X639 | 2.10 ± 0.06 k | 0.57 ± 0.06 jkl | 0.36 ± 0.04 ij | 81.00 ± 6.08 a | 0.30 ± 0.03 e–h | |
| Kirrumakki nucellar | 2.10 ± 0.04 k | 0.53 ± 0.06 kl | 0.41 ± 0.04 hij | 82.33 ± 2.31 a | 0.51 ± 0.01 ab | |
| Rangpur poona nucellar | 1.57 ± 0.04 m | 0.43 ± 0.06 l | 0.14 ± 0.03 k | 82.00 ± 1.73 a | 0.10 ± 0.00 kl | |
| p-value | ||||||
| pDrought | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| pRootstock | <0.0001 | <0.0001 | <0.0001 | =0.0261 | <0.0001 | |
| pDrought × Rootstock | <0.0001 | <0.0001 | <0.0001 | =0.0978 | <0.0001 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shafqat, W.; Mazrou, Y.S.A.; Sami-ur-Rehman; Nehela, Y.; Ikram, S.; Bibi, S.; Naqvi, S.A.; Hameed, M.; Jaskani, M.J. Effect of Three Water Regimes on the Physiological and Anatomical Structure of Stem and Leaves of Different Citrus Rootstocks with Distinct Degrees of Tolerance to Drought Stress. Horticulturae 2021, 7, 554. https://0-doi-org.brum.beds.ac.uk/10.3390/horticulturae7120554
Shafqat W, Mazrou YSA, Sami-ur-Rehman, Nehela Y, Ikram S, Bibi S, Naqvi SA, Hameed M, Jaskani MJ. Effect of Three Water Regimes on the Physiological and Anatomical Structure of Stem and Leaves of Different Citrus Rootstocks with Distinct Degrees of Tolerance to Drought Stress. Horticulturae. 2021; 7(12):554. https://0-doi-org.brum.beds.ac.uk/10.3390/horticulturae7120554
Chicago/Turabian StyleShafqat, Waqar, Yasser S. A. Mazrou, Sami-ur-Rehman, Yasser Nehela, Sufian Ikram, Sana Bibi, Summar A. Naqvi, Mansoor Hameed, and Muhammad Jafar Jaskani. 2021. "Effect of Three Water Regimes on the Physiological and Anatomical Structure of Stem and Leaves of Different Citrus Rootstocks with Distinct Degrees of Tolerance to Drought Stress" Horticulturae 7, no. 12: 554. https://0-doi-org.brum.beds.ac.uk/10.3390/horticulturae7120554