The Adductomics of Isolevuglandins: Oxidation of IsoLG Pyrrole Intermediates Generates Pyrrole–Pyrrole Crosslinks and Lactams
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Preparation of Iso[4]LGE2-Pyrrole under Anoxic Conditions
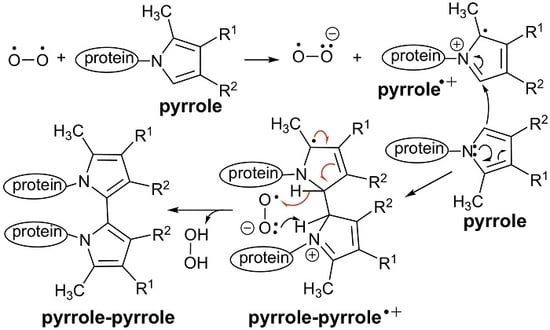
3.2. An Electron Transfer Mechanism for Pyrrole–Pyrrole Crosslinking by Oxygen
3.3. Reaction of Iso[4]LGE2-Pyrrole with Nα-acetylcysteine
3.4. Although Initially Slow, the Oxidation of Pure Iso[4]LGE2-Pyrrole with Air Accelerates.
3.5. Autoxidation of Iso[4]Lge2-Pyrrole is Promoted by Single Electron Transfer Catalysts.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Salomon, R.G. Levuglandins and isolevuglandins: Stealthy toxins of oxidative injury. Antioxid. Redox Signal. 2005, 7, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Jang, G.F.; Zhang, L.; Crabb, J.W.; Laird, J.; Linetsky, M.; Salomon, R.G. Molecular Structures of Isolevuglandin-Protein Crosslinks. Chem. Res. Toxicol. 2016, 29, 1628–1640. [Google Scholar] [CrossRef] [PubMed]
- Salomon, R.G.; Bi, W. Isolevuglandin adducts in disease. Antioxid. Redox Signal. 2015, 22, 1703–1718. [Google Scholar] [CrossRef]
- Charvet, C.; Liao, W.L.; Heo, G.Y.; Laird, J.; Salomon, R.G.; Turko, I.V.; Pikuleva, I.A. Isolevuglandins and mitochondrial enzymes in the retina: Mass spectrometry detection of post-translational modification of sterol-metabolizing CYP27A1. J. Biol. Chem. 2011, 286, 20413–20422. [Google Scholar] [CrossRef] [PubMed]
- Boutaud, O.; Andreasson, K.I.; Zagol-Ikapitte, I.; Oates, J.A. Cyclooxygenase-dependent lipid-modification of brain proteins. Brain Pathol. 2005, 15, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Bennett, B.J.; de Aguiar Vallim, T.Q.; Wang, Z.; Shih, D.M.; Meng, Y.; Gregory, J.; Allayee, H.; Lee, R.; Graham, M.; Crooke, R.; et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metabol. 2013, 17, 49–60. [Google Scholar] [CrossRef]
- Englander, S.W.; Calhoun, D.B.; Englander, J.J. Biochemistry without oxygen. Anal. Biochem. 1987, 161, 300–306. [Google Scholar] [CrossRef]
- Iyer, R.S.; Kobierski, M.E.; Salomon, R.G. Generation of Pyrroles in The Reaction of Levuglandin E2 with Proteins. J. Org. Chem. 1994, 59, 6038–6043. [Google Scholar] [CrossRef]
- Beaver, B.D.; Hazlett, R.N.; Cooney, J.V.; Watkins, J.M. A new look at the mechanisms of oxidative sediment formation in fuels. Fuel Sci. Technol. Int. 1988, 6, 131–150. [Google Scholar] [CrossRef]
- Zhu, M.; Spink, D.C.; Yan, B.; Bank, S.; DeCaprio, A.P. Inhibition of 2,5-hexanedione-induced protein crosslinking by biological thiols: Chemical mechanisms and toxicological implications. Chem. Res. Toxicol. 1995, 8, 764–771. [Google Scholar] [CrossRef]
- Zhu, M.; Spink, D.C.; Yan, B.; Bank, S.; De Caprio, A.P. Formation and structure of crosslinking and monomeric pyrrole autoxidation products in 2,5-hexanedione-treated amino acids, peptides, and protein. Chem. Res. Toxicol. 1994, 7, 551–558. [Google Scholar] [CrossRef]
- Deng, H.; Peljo, P.; Cortés-Salazar, F.; Ge, P.; Kontturi, K.; Girault, H.H. Oxygen and hydrogen peroxide reduction by 1,2-diferrocenylethane at a liquid/liquid interface. J. Electroanal. Chem. 2012, 681, 16–23. [Google Scholar] [CrossRef]
- Mezhuev, Y.O.; Korshak, Y.V.; Shtilman, M.I.; Piskareva, A.I. Activation parameters of single-electron transfer from pyrrole molecule to persulfate ion. (English). Izv, V.U.Z. Khim. Khim. Tekhnol. 2012, 55, 42–45. [Google Scholar]
- Barriga, S. 2,2,6,6-Tetramethylpiperidin-1-oxyl (TEMPO). Synletters 2001, 2001, 0563. [Google Scholar] [CrossRef]
- Lebedev, O.L.; Kazarnovskii, S.N. Catalytic oxidation of aliphatic amines with hydrogen peroxide. Zh. Obshch. Khim. 1960, 30, 1631–1635. [Google Scholar]
- Novak, I.; Harrison, L.J.; Kovac, B.; Pratt, L.M. Electronic structure of persistent radicals: Nitroxides. J. Org. Chem. 2004, 69, 7628–7634. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.-C.; Li, Y. Formation of nitroxide radicals from secondary amines and peracids: A peroxyl radical oxidation pathway derived from electron spin resonance detection and density functional theory calculation. J. Mol. Catal. A Chem. 2007, 271, 32–41. [Google Scholar] [CrossRef]
- Samuni, A.; Goldstein, S.; Russo, A.; Mitchell, J.B.; Krishna, M.C.; Neta, P. Kinetics and mechanism of hydroxyl radical and OH-adduct radical reactions with nitroxides and with their hydroxylamines. J. Am. Chem. Soc. 2002, 124, 8719–8724. [Google Scholar] [CrossRef] [PubMed]
- Fa, Z.; YouCheng, L. Electron transfer reactions of piperidine aminoxyl radicals. Chin. Sci. Bull. 2010, 55, 2760–2783. [Google Scholar]
- Bernoud-Hubac, N.; Fay, L.B.; Armarnath, V.; Guichardant, M.; Bacot, S.; Davies, S.S.; Roberts, L.J., 2nd; Lagarde, M. Covalent binding of isoketals to ethanolamine phospholipids. Free Radic. Biol. Med. 2004, 37, 1604–1611. [Google Scholar] [CrossRef] [PubMed]
- Franco, C.; Olmsted, J., 3rd. Photochemical determination of the solubility of oxygen in various media. Talanta 1990, 37, 905–909. [Google Scholar] [CrossRef]
- Battino, R. Oxygen and Ozone; Pergamon: Oxford, NY, USA, 1981; Volume 7. [Google Scholar]
- Murov, S.L. Handbook of Photochemistry; Dekker: New York, NY, USA, 1973. [Google Scholar]
- Achord, J.M.; Hussey, C.L. Determination of dissolved oxygen in nonaqueous electrochemical solvents. Anal. Biochem. 1980, 52, 601–602. [Google Scholar] [CrossRef]
- Tokunaga, J. Solubilities of oxygen, nitrogen, and carbon dioxide in aqueous alcohol solutions. J. Chem. Eng. Data 1975, 20, 41–46. [Google Scholar] [CrossRef]
- Monroe, B.M. Photochemical estimation of oxygen solubility. Photochem. Photobiol. 1982, 35, 863–865. [Google Scholar] [CrossRef]
- Evans, D.H.; Claiborne, J.B. The Physiology of Fishes; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Yuki, E. Effect of trimethylamine oxide on the oxidation of natural tocopherol mixtures. Yukagaku 1974, 23, 710–713. [Google Scholar]














© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, W.; Jang, G.-F.; Zhang, L.; Crabb, J.W.; Laird, J.; Linetsky, M.; Salomon, R.G. The Adductomics of Isolevuglandins: Oxidation of IsoLG Pyrrole Intermediates Generates Pyrrole–Pyrrole Crosslinks and Lactams. High-Throughput 2019, 8, 12. https://0-doi-org.brum.beds.ac.uk/10.3390/ht8020012
Bi W, Jang G-F, Zhang L, Crabb JW, Laird J, Linetsky M, Salomon RG. The Adductomics of Isolevuglandins: Oxidation of IsoLG Pyrrole Intermediates Generates Pyrrole–Pyrrole Crosslinks and Lactams. High-Throughput. 2019; 8(2):12. https://0-doi-org.brum.beds.ac.uk/10.3390/ht8020012
Chicago/Turabian StyleBi, Wenzhao, Geeng-Fu Jang, Lei Zhang, John W. Crabb, James Laird, Mikhail Linetsky, and Robert G. Salomon. 2019. "The Adductomics of Isolevuglandins: Oxidation of IsoLG Pyrrole Intermediates Generates Pyrrole–Pyrrole Crosslinks and Lactams" High-Throughput 8, no. 2: 12. https://0-doi-org.brum.beds.ac.uk/10.3390/ht8020012