A Diffusive Gradient-in-Thin-Film Technique for Evaluation of the Bioavailability of Cd in Soil Contaminated with Cd and Pb
Abstract
:1. Introduction
2. Materials and Methods
2.1. DGT Assembly and Preparation
2.2. Soil Samples and Plants
2.3. Pot Experiment
2.4. DGT Experiments
- (1)
- Pretreatment of the soil sample: each soil sample (80 g) was weighed in a 100 mL plastic container and mixed with deionized water to 40% maximum water holding capacity (MWHC); 48 h later, water was added to achieve 80% MWHC and the resulting slurries were allowed to equilibrate at ambient temperature for 24 h before DGT deployment.
- (2)
- DGT deployment: the assembled DGT devices were gently placed on the soil surface of each pot for 24 h, but the gel films were not squeezed. The containers were closed, and Petri dishes with wet cellulose were placed in the containers to retain the soil moisture. Three replicates per pot were kept at 25 °C for 24 h.
- (3)
- DGT retrieval and elution: after 24 h, all of the DGT devices were retrieved and rinsed with deionized water. The binding gel layers were removed from the DGT units, placed in polyethylene vials, and eluted in 1 mL of 1 mol/L HNO3 for 24 h. The Cd concentration in the extractant was determined by flame atomic adsorption spectrophotometry (Z-81001, Hitachi, Hitachi, Japan).
- (4)
- DGT calculation: The concentrations of Cd accumulated by the DGT devices were calculated according to Equation (1):
2.5. Soil Solution Concentration
2.6. Single-Solvent Process
2.7. Determination of Cd in Plants
3. Results and Discussion
3.1. Wheat and Maize Growth Response to Cd and Pb
3.2. Cd Uptake by Plants
3.3. DGT Measurement and Soil Solution Concentration
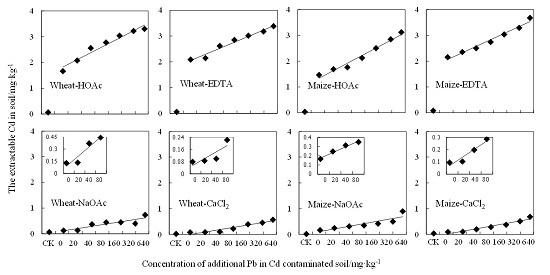
3.4. Extractable-Cd Determined by Single Extraction Methods
3.5. Comparison of DGT with Chemical Extraction for Cd Bioavailability
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- McLaughlin, M.J.; Parker, D.R.; Clarke, J.M. Metals and micronutrients—Food safety issues. Field Crop. Res. 1999, 60, 143–163. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Beryllium, Cadmium, Mercury, and Exposures in the Glass Manufacturing Industry; IARC; World Health Organization: Geneva, Switzerland, 1993. [Google Scholar]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol. 2011, 2011. [Google Scholar] [CrossRef]
- Sánchez-Martín, M.J.; García-Delgado, M.; Lorenzo, L.F.; Rodríguez-Cruz, M.S.; Arienzo, M. Heavy metals in sewage sludge amended soils determined by sequential extractions as a function of incubation time of soils. Geoderma 2007, 142, 262–273. [Google Scholar] [CrossRef]
- Ytreberg, E.; Karlsson, J.; Hoppe, S.; Eklund, B.; Ndungu, K. Effect of organic complexation on copper accumulation and toxicity to the estuarine Red Macroalga Ceramium tenuicorne: A test of the free ion activity model. Environ. Sci. Technol. 2011, 45, 3145–3153. [Google Scholar] [CrossRef] [PubMed]
- Houba, V.J.G.; Lexmond, T.M.; Novozamsky, I.; van der Lee, J.J. State of the art and future developments in soil analysis for bioavailability assessment. Sci. Total Environ. 1996, 178, 21–28. [Google Scholar] [CrossRef]
- Davlson, W.; Zhang, H. In situ speciation measurements of trace components in natural waters using thin-film gels. Nature 1994, 367, 546–548. [Google Scholar] [CrossRef]
- Jansen, B.; Nierop, K.G.; Verstraten, J.M. Influence of pH and metal/carbon ratios on soluble organic complexation of Fe(II), Fe(III) and Al(III) in soil solutions determined by diffusive gradients in thin films. Anal. Chim. Acta 2002, 454, 259–270. [Google Scholar] [CrossRef]
- Davison, W.; Fones, G.R.; Grime, G.W. Dissolved metals in surface sediment and a microbial mat at 100-μm resolution. Nature 1997, 387, 885–888. [Google Scholar] [CrossRef]
- DeVries, C.R.; Wang, F. In situ two-dimensional high-resolution profiling of sulfide in sediment interstitial waters. Environ. Sci. Technol. 2003, 37, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Omanović, D.; Pižeta, I.; Vukosav, P.; Kovács, E.; Frančišković-Bilinski, S.; Tamás, J. Assessing element distribution and speciation in a stream at abandoned Pb–Zn mining site by combining classical, in-situ DGT and modelling approaches. Sci. Total Environ. 2015, 511, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Davison, W.; Zhang, H. Progress in understanding the use of diffusive gradients in thin films (DGT)—Back to basics. Environ. Chem. 2012, 9, 1–13. [Google Scholar] [CrossRef]
- Davison, W.; Zhang, H.; Miller, S. Developing and applying new techniques for measuring steep chemical gradients of trace metals and inorganic ions at the sediment-water interface. Mineral. Mag. A 1994, 58, 215–216. [Google Scholar] [CrossRef]
- Zhang, H.; Davison, W.; Miller, S.; Tych, W. In situ high resolution measurements of fluxes of Ni, Cu, Fe, and Mn and concentrations of Zn and Cd in porewaters by DGT. Geochim. Cosmochim. Acta 1995, 59, 4181–4192. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, F.J.; Sun, B.; Davison, W.; Mcgrath, S.P. A new method to measure effective soil solution concentration predicts copper availability to plants. Environ. Sci. Technol. 2001, 35, 2602–2607. [Google Scholar] [CrossRef] [PubMed]
- Black, A.; McLaren, R.G.; Reichman, S.M.; Speir, T.W.; Condron, L.M. Evaluation of soil metal bioavailability estimates using two plant species (L. perenne and T. aestivum) grown in a range of agricultural soils treated with biosolids and metal salts. Environ. Pollut. 2011, 159, 1523–1535. [Google Scholar] [CrossRef] [PubMed]
- Pérez, A.L.; Anderson, K.A. DGT estimates cadmium accumulation in wheat and potato from phosphate fertilizer applications. Sci. Total Environ. 2009, 407, 5096–5103. [Google Scholar] [CrossRef] [PubMed]
- Tandy, S.; Mundus, S.; Yngvesson, J.; de Bang, T.C.; Lombi, E.; Schjørring, J.K.; Husted, S. The use of DGT for prediction of plant available copper, zinc and phosphorus in agricultural soils. Plant Soil 2011, 346, 167–180. [Google Scholar] [CrossRef]
- Qiu, H.; Gu, H.-H.; He, E.-R.; Wang, S.-H.; Qiu, R.-L. Attenuation of metal bioavailability in acidic multi-metal contaminated soil treated with fly ash and steel slag. Pedosphere 2012, 22, 544–553. [Google Scholar] [CrossRef]
- Song, J.; Zhao, F.J.; Luo, Y.M.; McGrath, S.P.; Zhang, H. Copper uptake by Elsholtzia splendens and Silene vulgaris and assessment of copper phytoavailability in contaminated soils. Environ. Pollut. 2004, 128, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lombi, E.; Smolders, E.; McGrath, S. Kinetics of Zn release in soils and prediction of Zn concentration in plants using diffusive gradients in thin films. Environ. Sci. Technol. 2004, 38, 3608–3613. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Disla, J.M.; Speir, T.W.; Gómez, I.; Clucas, L.M.; McLaren, R.G.; Navarro-Pedreño, J. Evaluation of different extraction methods for the assessment of heavy metal bioavailability in various soils. Water Air Soil Pollut. 2010, 213, 471–483. [Google Scholar] [CrossRef]
- Cornu, J.Y.; Denaix, L. Prediction of zinc and cadmium phytoavailability within a contaminated agricultural site using DGT. Environ. Chem. 2006, 3, 61–64. [Google Scholar] [CrossRef]
- Almås, Å.R.; Lombnæs, P.; Sogn, T.A.; Mulder, J. Speciation of Cd and Zn in contaminated soils assessed by DGT-DIFS, and WHAM/Model VI in relation to uptake by spinach and ryegrass. Chemosphere 2006, 62, 1647–1655. [Google Scholar]
- Nolan, A.L.; Zhang, H.; McLaughlin, M.J. Prediction of zinc, cadmium, lead, and copper availability to wheat in contaminated soils using chemical speciation, diffusive gradients in thin films, extraction, and isotopic dilution techniques. J. Environ. Qual. 2005, 34, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Davison, W. Performance characteristics of diffusion gradients in thin films for the in situ measurement of trace metals in aqueous solution. Anal. Chem. 1995, 67, 3391–3400. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, L.; Ding, S.; Li, C.; Yang, J.; Chen, J.; Wang, P. Evaluation of the diffusive gradients in thin films technique using a mixed binding gel for measuring iron, phosphorus and arsenic in the environment. Environ. Sci. Processes Impacts 2015, 17, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhang, H.; Zhao, F.J.; Davison, W. Distinguishing diffusional and plant control of Cd and Ni uptake by hyperaccumulator and nonhyperaccumulator plants. Environ. Sci. Technol. 2010, 44, 6636–6641. [Google Scholar] [CrossRef] [PubMed]
- Quevauviller, P. Operationally defined extraction procedures for soil and sediment analysis I. Standardization. TrAC Trends Anal. Chem. 1998, 17, 289–298. [Google Scholar] [CrossRef]
- Feng, M.H.; Shan, X.Q.; Zhang, S.Z.; Wen, B. Comparison of a rhizosphere-based method with other one-step extraction methods for assessing the bioavailability of soil metals to wheat. Chemosphere 2005, 59, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Novozamsky, I.; Lexmond, T.M.; Houba, V.J.G. A single extraction procedure of soil for evaluation of uptake of some heavy metals by plants. Int. J. Environ. Anal. Chem. 1993, 51, 47–58. [Google Scholar] [CrossRef]
- Kaplan, O.; Yaman, M.; Kaya, G. Distribution of nickel in different phases of soil samples and plant parts taken from serpentine and copper mining area. Asian J. Chem. 2009, 21, 5757–5767. [Google Scholar]
- Dahmani-Muller, H.; Van Oort, F.; Gelie, B.; Balabane, M. Strategies of heavy metal uptake by three plant species growing near a metal smelter. Environ. Pollut. 2000, 109, 231–238. [Google Scholar] [CrossRef]
- Harper, M.P.; Davison, W.; Zhang, H.; Tych, W. Kinetics of metal exchange between solids and solutions in sediments and soils interpreted from DGT measured fluxes. Geochim. Cosmochim. Acta 1998, 62, 2757–2770. [Google Scholar] [CrossRef]
- Forsberg, L.S.; Kleja, D.B.; Greger, M.; Ledin, S. Effects of sewage sludge on solution chemistry and plant uptake of Cu in sulphide mine tailings at different weathering stages. Appl. Geochem. 2009, 24, 475–482. [Google Scholar] [CrossRef]
- Lin, Q.; Chen, Y.X.; Chen, H.M.; Yu, Y.L.; Luo, Y.M.; Wong, M.H. Chemical behavior of Cd in rice rhizosphere. Chemosphere 2003, 50, 755–761. [Google Scholar] [CrossRef]
- Paya-Perez, A.; Sala, J.; Mousty, F. Comparison of ICP-AES and ICP-MS for the analysis of trace elements in soil extracts. Int. J. Environ. Anal. Chem. 1993, 51, 223–230. [Google Scholar] [CrossRef]
- McLaughlin, M.J.; Zarcinas, B.A.; Stevens, D.P.; Cook, N. Soil testing for heavy metals. Commun. Soil Sci. Plant Anal. 2000, 31, 1661–1700. [Google Scholar] [CrossRef]
- Kim, K.R.; Owens, G.; Naidu, R. Heavy metal distribution, bioaccessibility and phytoavailability in long-term contaminated soils from Lake Macquarie, Australia. Aust. J. Soil Res. 2009, 47, 166–176. [Google Scholar] [CrossRef]
- McBride, M.B. Environmental Chemistry of Soils; Oxford University Press: New York, NY, USA, 1994. [Google Scholar]
- Manouchehri, N.; Besançon, S.; Bermond, A. Kinetic characterizing of soil trace metal availability using Soil/EDTA/Chelex mixture. Chemosphere 2011, 83, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wang, X.; Luo, J.; Yu, H.; Zhang, H. Evaluation of holistic approaches to predicting the concentrations of metals in field-cultivated rice. Environ. Sci. Technol. 2008, 42, 7649–7654. [Google Scholar] [CrossRef] [PubMed]
- Dočekalová, H.; Škarpa, P.; Dočekal, B. Diffusive gradient in thin films technique for assessment of cadmium and copper bioaccessibility to radish (Raphanus sativus). Talanta 2015, 134, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Bade, R.; Oh, S.; Shin, W.S. Diffusive gradients in thin films (DGT) for the prediction of bioavailability of heavy metals in contaminated soils to earthworm (Eisenia foetida) and oral bioavailable concentrations. Sci. Total Environ. 2012, 416, 127–136. [Google Scholar] [CrossRef] [PubMed]




| Extractant | Procedure | References |
|---|---|---|
| EDTA | 2.0 g of soil was extracted with 20 mL of 0.05 mol·L−1 EDTA adjusted using an ammonia solution to pH = 7.0 and shaken for 2 h | Feng et al. [30] |
| HOAc | 0.5 g of soil was extracted with 20 mL of 0.11 mol·L−1 HOAc and shaken for 16 h (overnight) | Quevauviller [29] |
| NaOAc | 4.0 g of soil was extracted with 20 mL of 1 mol·L−1 NaOAc and shaken for 2 h | Kaplan et al. [32] |
| CaCl2 | 2.0 g of soil was extracted with 20 mL of 0.01 mol·L−1 CaCl2 and shaken for 3 h | Novozamsky et al. [31] |
| Plant Species | Plant Tissues | CDGT | Csol | HOAc | EDTA | NaOAc | CaCl2 |
|---|---|---|---|---|---|---|---|
| Wheat | Shoot | 0.944 ** | 0.923 ** | 0.850 ** | 0.841 ** | 0.882 ** | 0.960 ** |
| Root | 0.931 ** | 0.905 ** | 0.789 ** | 0.763 ** | 0.857 ** | 0.943 ** | |
| Maize | Shoot | 0.994 ** | 0.971 ** | 0.899 ** | 0.900 ** | 0.891 ** | 0.925 ** |
| Root | 0.915 ** | 0.968 ** | 0.874 ** | 0.829 ** | 0.801 ** | 0.901 ** |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Wang, T.; Yao, Y.; Wang, C.; Liu, C.; Yuan, Y. A Diffusive Gradient-in-Thin-Film Technique for Evaluation of the Bioavailability of Cd in Soil Contaminated with Cd and Pb. Int. J. Environ. Res. Public Health 2016, 13, 556. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph13060556
Wang P, Wang T, Yao Y, Wang C, Liu C, Yuan Y. A Diffusive Gradient-in-Thin-Film Technique for Evaluation of the Bioavailability of Cd in Soil Contaminated with Cd and Pb. International Journal of Environmental Research and Public Health. 2016; 13(6):556. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph13060556
Chicago/Turabian StyleWang, Peifang, Teng Wang, Yu Yao, Chao Wang, Cui Liu, and Ye Yuan. 2016. "A Diffusive Gradient-in-Thin-Film Technique for Evaluation of the Bioavailability of Cd in Soil Contaminated with Cd and Pb" International Journal of Environmental Research and Public Health 13, no. 6: 556. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph13060556