Impact of an Extreme Winter Storm Event on the Coagulation/Flocculation Processes in a Prototype Surface Water Treatment Plant: Causes and Mitigating Measures
Abstract
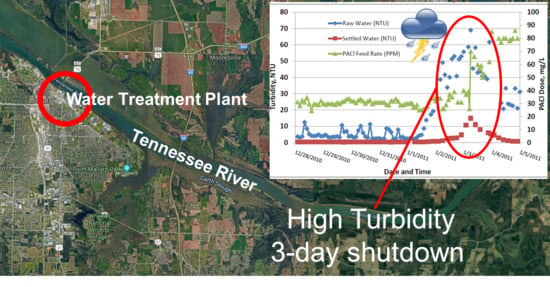
:1. Introduction
2. Materials and Methods
2.1. Raw Water and Sediments
2.2. Chemical Reagents
2.3. DOM Extraction
2.4. Jar Tests on Turbidity Removal
2.5. Effects of Temperature
2.6. Effects of DOM
2.7. Pre-Coagulation DOM Removal and Uses of Alternative Coagulants
2.8. Analytical Methods
3. Results and Discussion
3.1. Coagulation and Sedimentation of Various Sediment Particles
3.2. Effects of Temperature on Turbidity Removal
3.3. Effects of NOM on Coagulation/Flocculation
3.4. Measures to Cope with Turbidity Upset Due to Elevated NOM Shock Loading
4. Conclusions
- The key factor causing the DWTP turbidity treatment failure was the suddenly elevated NOM associated with some of the upstream sediment that was washed off by the severe winter storm into the river water. The low temperature had some minor adverse effects on the coagulation, but was not the main cause;
- Based on the plant data and our simulated experimental results, the native NOM at 6.14 mg·L−1 as TOC combined with the low temperature (7 °C) caused the sudden turbidity rise in the treated water and the process failure during the January winter storm;
- Jar tests using normal river water and river water modified with local sediment and DOM indicated that increasing the PACl dosage based on the raw water TOC level is a viable and practically feasible approach to cope with the shock changes. We recommend that the coagulant dosage should be based on the TOC level. As such, an optimal PACl dosage range of 22 kg-PACl/kg-TOC (under normal conditions) to 19 kg-PACl/kg-TOC (during storm time) or 75–90 mg·L−1 is recommended;
- For the DWTP NOM, the coagulation with PACl removed a fixed fraction (~20%) of the initial DOM, regardless of the coagulant dosage, indicating most of the NOM was less adsorbable or more soluble and may easily reach the final disinfection stage raising the concern of DBPs;
- The addition of chlorine and/or permanganate before coagulation was able to enhance the TOC removal, improve the turbidity removal by coagulation, and thus lower the coagulant demand. In particular, permanganate appears more cost-effective than NaOCl and does not cause DBPs. Consequently, a sample reference dosage of 0.29 kg-NaMnO4/kg-TOC and 19 kg-PACl/kg-TOC is recommended to cope with future storm water impacts in either winter or summer time. To facilitate the TOC-based chemical dosage adjustment, the variation of critical raw water quality parameters should be monitored closely, especially when severe weather is expected. The most critical parameters are raw water TOC and turbidity, in addition, SUVA, pH, and color should also be considered to determine the optimum dosage under specific conditions.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kistin, E.J.; Fogarty, J.; Pokrasso, R.S.; McCally, M.; McCornick, P.G. Climate change, water resources and child health. Arch. Dis. Child 2010, 95, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Manning, A.H.; Verplanck, P.L.; Caine, J.S.; Todd, A.S. Links between climate change, water-table depth, and water chemistry in a mineralized mountain watershed. Appl. Geochem. 2013, 37, 64–78. [Google Scholar] [CrossRef]
- Milly, P.C.D.; Wetherald, R.T.; Dunne, K.A.; Delworth, T.L. Increasing risk of great floods in a changing climate. Nature 2002, 415, 514–517. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.M. The impacts of global climate change on water treatment design and operations. In Securing Water and Wastewater Systems: Global Experiences; Clark, R.M., Hakim, S., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 251–272. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, P.F.; Wang, C.; Wang, X.; Hu, B. Assessment of the multi-objective reservoir operation for maintaining the turbidity maximum zone in the Yangtze River estuary. Int. J. Environ. Res. Public Health 2018, 15, 2118. [Google Scholar] [CrossRef] [PubMed]
- Hurst, A.M.; Edwards, M.J.; Chipps, M.; Jefferson, B.; Parsons, S.A. The impact of rainstorm events on coagulation and clarifier performance in potable water treatment. Sci. Total Environ. 2004, 321, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Inam, M.A.; Khan, R.; Park, D.R.; Khan, S.; Uddin, A.; Yeom, I.T. Complexation of antimony with natural organic matter: Performance evaluation during coagulation-flocculation process. Int. J. Environ. Res. Public Health 2019, 16, 1092. [Google Scholar] [CrossRef] [PubMed]
- Sharp, E.L.; Parsons, S.A.; Jefferson, B. Seasonal variations in natural organic matter and its impact on coagulation in water treatment. Sci. Total Environ. 2006, 363, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Abebe, L.S.; Chen, X.Y.; Sobsey, M.D. Chitosan coagulation to improve microbial and turbidity removal by ceramic water filtration for household drinking water treatment. Int. J. Environ. Res. Public Health 2016, 13, 269. [Google Scholar] [CrossRef]
- Nefale, A.D.; Kamika, I.; Obi, C.L.; Momba, M.N.B. The limpopo non-metropolitan drinking water supplier response to a diagnostic tool for technical compliance. Int. J. Environ. Res. Public Health 2017, 14, 810. [Google Scholar] [CrossRef]
- Van Benschoten, J.E.; Edzwald, J.K. Chemical aspects of coagulation using aluminum salts—I. Hydrolytic reactions of alum and polyaluminum chloride. Water Res. 1990, 24, 1519–1526. [Google Scholar] [CrossRef]
- Xiao, F.; Zhang, B.; Lee, C. Effects of low temperature on aluminum(III) hydrolysis: Theoretical and experimental studies. J. Environ. Sci. 2008, 20, 907–914. [Google Scholar] [CrossRef]
- Morris, J.K.; Knocke, W.R. Temperature effects on the use of metal-ion coagulants for water treatment. J. Am. Water Work. Assoc. 1984, 76, 74–79. [Google Scholar] [CrossRef]
- Duan, J.; Wang, J.; Graham, N.; Wilson, F. Coagulation of humic acid by aluminium sulphate in saline water conditions. Desalination 2002, 150, 1–14. [Google Scholar] [CrossRef]
- Libecki, B.; Dziejowski, J. Optimization of Humic Acids Coagulation with Aluminum and Iron(III) Salts. Pol. J. Environ. Stud. 2008, 17, 397–403. [Google Scholar]
- Wei, L.; Yong-Mei, L. Use of ferrate pre-oxidation in enhancing the treatment of NOM-rich lake waters. Water Supply 2004, 4, 121–128. [Google Scholar] [CrossRef]
- Wilkinson, K.J.; Negre, J.C.; Buffle, J. Coagulation of colloidal material in surface waters: The role of natural organic matter. J. Contam. Hydrol. 1997, 26, 229–243. [Google Scholar] [CrossRef]
- Kretzschmar, R.; Sticher, H.; Hesterberg, D. Effects of adsorbed humic acid on surface charge and flocculation of kaolinite. Soil Sci. Soc. Am. J. 1997, 61, 101–108. [Google Scholar] [CrossRef]
- Świetlik, J.; Dąbrowska, A.; Raczyk-Stanisławiak, U.; Nawrocki, J. Reactivity of natural organic matter fractions with chlorine dioxide and ozone. Water Res. 2004, 38, 547–558. [Google Scholar] [CrossRef]
- Randtke, S.J. Organic contaminant removal by coagulation and related process combinations. J. Am. Water Work. Assoc. 1988, 80, 40–56. [Google Scholar] [CrossRef]
- Edzwald, J.K.; Becker, W.C.; Wattier, K.L. Surrogate parameters for monitoring organic matter and THM precursors. J. Am. Water Work. Assoc. 1985, 77, 122–132. [Google Scholar] [CrossRef]
- Pernitsky, D.J.; Edzwald, J.K. Selection of alum and polyaluminum coagulants: Principles and applications. J. Water Supply Res. Technol. 2006, 55, 121–141. [Google Scholar] [CrossRef]
- Rebhun, M.; Lurie, M. Control of organic matter by coagulation and floc separation. Water Sci. Technol. 1993, 27, 1–20. [Google Scholar] [CrossRef]
- Ma, J.; Li, G.B.; Chen, Z.L.; Xu, G.R.; Cai, G.Q. Enhanced coagulation of surface waters with high organic content by permangante preoxidation. Water Supply 2001, 1, 51–61. [Google Scholar] [CrossRef]
- Liu, R.; Liu, H.; Qiang, Z.; Qu, J.; Li, G.; Wang, D. Effects of calcium ions on surface characteristics and adsorptive properties of hydrous manganese dioxide. J. Colloid Interf. Sci. 2009, 331, 275–280. [Google Scholar] [CrossRef] [PubMed]








| Item | Value | Item | Level (mg·L−1) |
|---|---|---|---|
| pH | 7.52 | Total Organic Carbon (TOC) | 1.30 |
| Turbidity (NTU) | 3.57 | Hardness as CaCO3 | 62.30 |
| Alkalinity as CaCO3 | 69.00 | ||
| Ca | 18.00 | ||
| Mg | 5.62 |
| Dosage (mg·L−1) | SS1 | SS2 | SS3 | SS4 | SS5 | SS6 | SS7 | SS8 | RBS3 | RBS5 | RBS6 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 30 | 0.12 | 0.11 | 0.14 | 0.12 | 0.13 | 0.21 | 0.17 | 0.17 | 0.14 | 0.13 | 0.15 |
| 45 | 0.16 | 0.10 | 0.12 | 0.09 | 0.12 | 0.11 | 0.10 | 0.18 | 0.12 | 0.12 | 0.10 |
| Dosage (mg·L−1) | RBS7 | RBS8 | RBS9 | RBS10 | RBS11 | RBS12 | RBS13 | RBS14 | RBS15 | RWI | RWC |
| 30 | 0.16 | 0.14 | 0.22 | 0.14 | 0.14 | 0.20 | 0.15 | 0.10 | 0.12 | 0.32 | 0.17 |
| 45 | 0.15 | 0.11 | 0.19 | 0.13 | 0.12 | 0.20 | 0.14 | 0.09 | 0.16 | 0.21 | 0.16 |
| SUVA | Composition | Coagulation |
|---|---|---|
| <2 | Mostly non-humics Low hydrophobicity Low molecular weight | NOM has little influence Poor TOC removal |
| 2–4 | Mixture of aquatic humics and other NOM Mixture of hydrophobic and hydrophilic NOM Mixture of molecular weights | NOM influences Fair to good TOC removal |
| >4 | Mostly aquatic humics High hydrophobicity High molecular weight | NOM controls Good TOC removal |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, F.; Lv, H.; Zhao, X.; Zhao, D. Impact of an Extreme Winter Storm Event on the Coagulation/Flocculation Processes in a Prototype Surface Water Treatment Plant: Causes and Mitigating Measures. Int. J. Environ. Res. Public Health 2019, 16, 2808. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16152808
Qiu F, Lv H, Zhao X, Zhao D. Impact of an Extreme Winter Storm Event on the Coagulation/Flocculation Processes in a Prototype Surface Water Treatment Plant: Causes and Mitigating Measures. International Journal of Environmental Research and Public Health. 2019; 16(15):2808. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16152808
Chicago/Turabian StyleQiu, Fuguo, Huadong Lv, Xiao Zhao, and Dongye Zhao. 2019. "Impact of an Extreme Winter Storm Event on the Coagulation/Flocculation Processes in a Prototype Surface Water Treatment Plant: Causes and Mitigating Measures" International Journal of Environmental Research and Public Health 16, no. 15: 2808. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph16152808