Interval Hypoxic Training Enhances Athletic Performance and Does Not Adversely Affect Immune Function in Middle- and Long-Distance Runners
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.3. Blood Composition
2.4. Hemodynamic Function
2.5. ANS Function
2.6. Immune Function
2.7. Athletic Performance
2.8. Statistical Analysis
3. Results
3.1. Body Composition
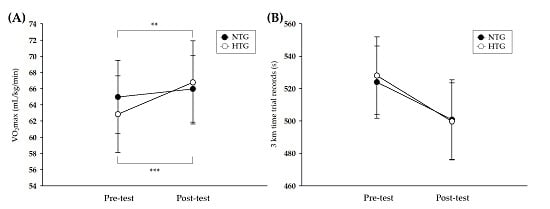
3.2. Athletic Performance
3.3. Hemodynamic Function
3.4. ANS Function
3.5. Immune Function
4. Discussion
4.1. Athletic Performance
4.2. Hemodynamic Function
4.3. ANS Function
4.4. Immune Function
5. Limitation of the Study
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Park, H.Y.; Shin, C.; Lim, K. Intermittent hypoxic training for 6 weeks in 3000 m hypobaric hypoxia conditions enhances exercise economy and aerobic exercise performance in moderately trained swimmers. Biol. Sport 2018, 35, 49–56. [Google Scholar] [CrossRef]
- Im, J.Y.; Bang, H.S.; Seo, D.Y. The Effects of 12 Weeks of a Combined Exercise Program on Physical Function and Hormonal Status in Elderly Korean Women. Int. J. Environ. Res. Public Health 2019, 16. [Google Scholar] [CrossRef] [Green Version]
- Park, H.Y.; Hwang, H.; Park, J.; Lee, S.; Lim, K. The effects of altitude/hypoxic training on oxygen delivery capacity of the blood and aerobic exercise capacity in elite athletes—A meta-analysis. J. Exerc. Nutr. Biochem. 2016, 20, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Millet, G.P.; Faiss, R.; Brocherie, F.; Girard, O. Hypoxic training and team sports: A challenge to traditional methods? Br. J. Sports Med. 2013, 47, i6–i7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.W.; Jung, W.S.; Park, W. Twelve Weeks of Combined Resistance and Aerobic Exercise Improves Cardiometabolic Biomarkers and Enhances Red Blood Cell Hemorheological Function in Obese Older Men: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2019, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, W.S.; Hwang, H.; Kim, J.; Park, H.Y.; Lim, K. Effect of interval exercise versus continuous exercise on excess post-exercise oxygen consumption during energy-homogenized exercise on a cycle ergometer. J. Exerc. Nutr. Biochem. 2019, 23, 45–50. [Google Scholar] [CrossRef]
- Nystoriak, M.A.; Bhatnagar, A. Cardiovascular Effects and Benefits of Exercise. Front. Cardiovasc. Med. 2018, 5, 135. [Google Scholar] [CrossRef] [Green Version]
- Park, H.Y.; Lim, K. Effects of Hypoxic Training versus Normoxic Training on Exercise Performance in Competitive Swimmers. J. Sports Sci. Med. 2017, 16, 480–488. [Google Scholar]
- Faiss, R.; Girard, O.; Millet, G.P. Advancing hypoxic training in team sports: From intermittent hypoxic training to repeated sprint training in hypoxia. Br. J. Sports Med. 2013, 47, i45–i50. [Google Scholar] [CrossRef] [Green Version]
- Girard, O.; Brocherie, F.; Millet, G.P. Effects of Altitude/Hypoxia on Single- and Multiple-Sprint Performance: A Comprehensive Review. Sports Med. 2017, 47, 1931–1949. [Google Scholar] [CrossRef]
- Hamlin, M.J.; Marshall, H.C.; Hellemans, J.; Ainslie, P.N.; Anglem, N. Effect of intermittent hypoxic training on 20 km time trial and 30 s anaerobic performance. Scand. J. Med. Sci. Sports 2010, 20, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, L. Interval hypoxic training. Adv. Exp. Med. Biol. 2001, 502, 377–399. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Jung, W.S.; Kim, J.; Hwang, H.; Lim, K. Efficacy of intermittent hypoxic training on hemodynamic function and exercise performance in competitive swimmers. J. Exerc. Nutr. Biochem. 2018, 22, 32–38. [Google Scholar] [CrossRef]
- Czuba, M.; Waskiewicz, Z.; Zajac, A.; Poprzecki, S.; Cholewa, J.; Roczniok, R. The effects of intermittent hypoxic training on aerobic capacity and endurance performance in cyclists. J. Sports Sci. Med. 2011, 10, 175–183. [Google Scholar]
- Geiser, J.; Vogt, M.; Billeter, R.; Zuleger, C.; Belforti, F.; Hoppeler, H. Training high-living low: Changes of aerobic performance and muscle structure with training at simulated altitude. Int. J. Sports Med. 2001, 22, 579–585. [Google Scholar] [CrossRef] [Green Version]
- Beidleman, B.A.; Muza, S.R.; Fulco, C.S.; Jones, J.E.; Lammi, E.; Staab, J.E.; Cymerman, A. Intermittent hypoxic exposure does not improve endurance performance at altitude. Med. Sci. Sports Exerc. 2009, 41, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Katayama, K.; Sato, K.; Matsuo, H.; Ishida, K.; Iwasaki, K.; Miyamura, M. Effect of intermittent hypoxia on oxygen uptake during submaximal exercise in endurance athletes. Eur. J. Appl. Physiol. 2004, 92, 75–83. [Google Scholar] [CrossRef]
- Rodriguez, F.A.; Truijens, M.J.; Townsend, N.E.; Stray-Gundersen, J.; Gore, C.J.; Levine, B.D. Performance of runners and swimmers after four weeks of intermittent hypobaric hypoxic exposure plus sea level training. J. Appl. Physiol. 2007, 103, 1523–1535. [Google Scholar] [CrossRef]
- Roels, B.; Bentley, D.J.; Coste, O.; Mercier, J.; Millet, G.P. Effects of intermittent hypoxic training on cycling performance in well-trained athletes. Eur. J. Appl. Physiol. 2007, 101, 359–368. [Google Scholar] [CrossRef]
- Park, H.Y.; Jung, W.S.; Kim, J.; Lim, K. Twelve weeks of exercise modality in hypoxia enhances health-related function in obese older Korean men: A randomized controlled trial. Geriatr. Gerontol. Int. 2019, 19, 311–316. [Google Scholar] [CrossRef]
- Vitale, J.A.; Bonato, M. Heart Rate Variability in Sport Performance: Do Time of Day and Chronotype Play A Role? J. Clin. Med. 2019, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herzig, D.; Asatryan, B.; Brugger, N.; Eser, P.; Wilhelm, M. The Association Between Endurance Training and Heart Rate Variability: The Confounding Role of Heart Rate. Front. Physiol. 2018, 9, 756. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.G. The role of heart rate variability in sports physiology. Exp. Ther. Med. 2016, 11, 1531–1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzeo, R.S.; Donovan, D.; Fleshner, M.; Butterfield, G.E.; Zamudio, S.; Wolfel, E.E.; Moore, L.G. Interleukin-6 response to exercise and high-altitude exposure: Influence of alpha-adrenergic blockade. J. Appl. Physiol. 2001, 91, 2143–2149. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, R.S. Physiological responses to exercise at altitude: An update. Sports Med. 2008, 38, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Beidleman, B.A.; Muza, S.R.; Fulco, C.S.; Cymerman, A.; Staab, J.E.; Sawka, M.N.; Lewis, S.F.; Skrinar, G.S. White blood cell and hormonal responses to 4300 m altitude before and after intermittent altitude exposure. Clin. Sci. 2006, 111, 163–169. [Google Scholar] [CrossRef] [Green Version]
- Mazzeo, R.S.; Child, A.; Butterfield, G.E.; Braun, B.; Rock, P.B.; Wolfel, E.E.; Zamudio, S.; Moore, L.G. Sympathoadrenal responses to submaximal exercise in women after acclimatization to 4300 meters. Metab. Clin. Exp. 2000, 49, 1036–1042. [Google Scholar] [CrossRef]
- Hartmann, G.; Tschop, M.; Fischer, R.; Bidlingmaier, C.; Riepl, R.; Tschop, K.; Hautmann, H.; Endres, S.; Toepfer, M. High altitude increases circulating interleukin-6, interleukin-1 receptor antagonist and C-reactive protein. Cytokine 2000, 12, 246–252. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Steensberg, A. Exercise and hypoxia: Effects on leukocytes and interleukin-6-shared mechanisms? Med. Sci. Sports Exerc. 2002, 34, 2004–2013. [Google Scholar] [CrossRef]
- Thake, C.D.; Mian, T.; Garnham, A.W.; Mian, R. Leukocyte counts and neutrophil activity during 4 h of hypocapnic hypoxia equivalent to 4000 m. Aviat. Space Environ. Med. 2004, 75, 811–817. [Google Scholar]
- Lee, Y.I.; Leem, Y.H. Acid sphingomyelinase inhibition alleviates muscle damage in gastrocnemius after acute strenuous exercise. J. Exerc. Nutr. Biochem. 2019, 23, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wilber, R.L. Application of altitude/hypoxic training by elite athletes. Med. Sci. Sports Exerc. 2007, 39, 1610–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.Y.; Kim, S.; Kim, Y.; Park, S.; Nam, S.S. Effects of exercise training at lactate threshold and detraining for 12 weeks on body composition, aerobic performance, and stress related variables in obese women. J. Exerc. Nutr. Biochem. 2019, 23, 22–28. [Google Scholar] [CrossRef] [PubMed]
- McLean, B.D.; Gore, C.J.; Kemp, J. Application of ’live low-train high’ for enhancing normoxic exercise performance in team sport athletes. Sports Med. 2014, 44, 1275–1287. [Google Scholar] [CrossRef] [PubMed]
- Roels, B.; Millet, G.P.; Marcoux, C.J.; Coste, O.; Bentley, D.J.; Candau, R.B. Effects of hypoxic interval training on cycling performance. Med. Sci. Sports Exerc. 2005, 37, 138–146. [Google Scholar] [CrossRef]
- Green, H.J.; Roy, B.; Grant, S.; Hughson, R.; Burnett, M.; Otto, C.; Pipe, A.; McKenzie, D.; Johnson, M. Increases in submaximal cycling efficiency mediated by altitude acclimatization. J. Appl. Physiol. 2000, 89, 1189–1197. [Google Scholar] [CrossRef] [Green Version]
- Saunders, P.U.; Telford, R.D.; Pyne, D.B.; Cunningham, R.B.; Gore, C.J.; Hahn, A.G.; Hawley, J.A. Improved running economy in elite runners after 20 days of simulated moderate-altitude exposure. J. Appl. Physiol. 2004, 96, 931–937. [Google Scholar] [CrossRef]
- Gore, C.J.; Hahn, A.G.; Aughey, R.J.; Martin, D.T.; Ashenden, M.J.; Clark, S.A.; Garnham, A.P.; Roberts, A.D.; Slater, G.J.; McKenna, M.J. Live high:Train low increases muscle buffer capacity and submaximal cycling efficiency. Acta Physiol. Scand. 2001, 173, 275–286. [Google Scholar] [CrossRef] [Green Version]
- Park, H.Y.; Park, W.; Lim, K. Living High-Training Low for 21 Days Enhances Exercise Economy, Hemodynamic Function, and Exercise Performance of Competitive Runners. J. Sports Sci. Med. 2019, 18, 427–437. [Google Scholar]
- Kim, J.; Park, H.Y.; Lim, K. Effects of 12 Weeks of Combined Exercise on Heart Rate Variability and Dynamic Pulmonary Function in Obese and Elderly Korean Women. Iran. J. Public Health 2018, 47, 74–81. [Google Scholar]
- Alansare, A.; Alford, K.; Lee, S.; Church, T.; Jung, H.C. The Effects of High-Intensity Interval Training vs. Moderate-Intensity Continuous Training on Heart Rate Variability in Physically Inactive Adults. Int. J. Environ. Res. Public Health 2018, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plews, D.J.; Laursen, P.B.; Stanley, J.; Kilding, A.E.; Buchheit, M. Training adaptation and heart rate variability in elite endurance athletes: Opening the door to effective monitoring. Sports Med. 2013, 43, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Hautala, A.; Tulppo, M.P.; Makikallio, T.H.; Laukkanen, R.; Nissila, S.; Huikuri, H.V. Changes in cardiac autonomic regulation after prolonged maximal exercise. Clin. Physiol. 2001, 21, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Lucini, D.; Norbiato, G.; Clerici, M.; Pagani, M. Hemodynamic and autonomic adjustments to real life stress conditions in humans. Hypertension 2002, 39, 184–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Licht, C.M.; de Geus, E.J.; van Dyck, R.; Penninx, B.W. Association between anxiety disorders and heart rate variability in The Netherlands Study of Depression and Anxiety (NESDA). Psychosom. Med. 2009, 71, 508–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kemp, A.H.; Quintana, D.S.; Felmingham, K.L.; Matthews, S.; Jelinek, H.F. Depression, comorbid anxiety disorders, and heart rate variability in physically healthy, unmedicated patients: Implications for cardiovascular risk. PLoS ONE 2012, 7, e30777. [Google Scholar] [CrossRef]
- Francis, J.L.; Weinstein, A.A.; Krantz, D.S.; Haigney, M.C.; Stein, P.K.; Stone, P.H.; Gottdiener, J.S.; Kop, W.J. Association between symptoms of depression and anxiety with heart rate variability in patients with implantable cardioverter defibrillators. Psychosom. Med. 2009, 71, 821–827. [Google Scholar] [CrossRef] [Green Version]
- Pyne, D.V.; McDonald, W.A.; Morton, D.S.; Swigget, J.P.; Foster, M.; Sonnenfeld, G.; Smith, J.A. Inhibition of interferon, cytokine, and lymphocyte proliferative responses in elite swimmers with altitude exposure. J. Interferon Cytokine Res. 2000, 20, 411–418. [Google Scholar] [CrossRef]
- Tiollier, E.; Schmitt, L.; Burnat, P.; Fouillot, J.P.; Robach, P.; Filaire, E.; Guezennec, C.; Richalet, J.P. Living high-training low altitude training: Effects on mucosal immunity. Eur. J. Appl. Physiol. 2005, 94, 298–304. [Google Scholar] [CrossRef]
- Brugniaux, J.V.; Schmitt, L.; Robach, P.; Jeanvoine, H.; Zimmermann, H.; Nicolet, G.; Duvallet, A.; Fouillot, J.P.; Richalet, J.P. Living high-training low: Tolerance and acclimatization in elite endurance athletes. Eur. J. Appl. Physiol. 2006, 96, 66–77. [Google Scholar] [CrossRef]
- Park, H.Y.; Jung, W.S.; Kim, J.; Hwang, H.; Kim, S.W.; An, Y.; Lee, H.; Jeon, S.; Lim, K. Effects of 2-Week Exercise Training in Hypobaric Hypoxic Conditions on Exercise Performance and Immune Function in Korean National Cycling Athletes with Disabilities: A Case Report. Int. J. Environ. Res. Public Health 2020, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Variables | NTG | HTG | t-Value | p-Value |
|---|---|---|---|---|
| Number (n) | n = 10 | n = 10 | - | - |
| Environmental condition (mmHg) | Sea level (760 mmHg) | 3000-m simulated altitude (526 mmHg) | - | - |
| Age (year) | 25.9 ± 1.2 | 26.3 ± 1.5 | −0.499 | 0.624 |
| Height (cm) | 176.9 ± 7.6 | 178.2 ± 3.5 | −0.514 | 0.616 |
| Weight (kg) | 70.8 ± 5.8 | 71.2 ± 6.3 | −0.490 | 0.630 |
| BMI (kg/m2) | 23.1 ± 1.5 | 22.8 ± 0.9 | −1.554 | 0.138 |
| FFM (kg) | 51.1 ± 4.4 | 52.1 ± 4.8 | −0.490 | 0.630 |
| Percent body fat (%) | 17.5 ± 2.7 | 18.4 ± 1.8 | −0.882 | 0.389 |
| Variables | NTG (n = 10) | p-Value | HTG (n = 10) | p-Value | p-Value (ɳ2) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Group | Time | Interaction | |||
| Weight (kg) | 70.8 ± 5.8 | 71.1 ± 5.8 | 0.146 | 71.2 ± 6.3 | 71.1 ± 7.3 | 0.945 | 0.555 (0.020) | 0.233 (0.078) | 0.269 (0.067) |
| BMI (kg/m2) | 23.1 ± 1.5 | 23.1 ± 1.6 | 0.105 | 22.8 ± 0.9 | 22.6 ± 0.9 | 0.390 | 0.136 (0.119) | 0.138 (0.118) | 0.919 (0.001) |
| FFM (kg) | 51.1 ± 4.4 | 50.6 ± 4.4 | 0.147 | 52.1 ± 4.8 | 52.1 ± 5.5 | 0.952 | 0.555 (0.020) | 0.233 (0.078) | 0.269 (0.067) |
| Percent body fat (%) | 17.5 ± 2.7 | 17.3 ± 2.9 | 0.274 | 18.4 ± 1.8 | 17.8 ± 2.0 | 0.275 | 0.477 (0.028) | 0.144 (0.115) | 0.549 (0.020) |
| Variables | NTG (n = 10) | p-Value | HTG (n = 10) | p-Value | p-Value (ɳ2) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Group | Time | Interaction | |||
| HR (beat/30 min) | 5375.7 ± 431.4 | 4946.1 ± 287.8 | 0.003 | 5207.4 ± 308.7 | 4830.5 ± 267.6 | 0.001 | 0.296 (0.060) | 0.000 (0.674) ††† | 0.694 (0.009) |
| VO2 (mL/30 min) | 1355.6 ± 114.4 | 1322.9 ± 118.9 | 0.094 | 1194.1 ± 139.5 | 1118.1 ± 141.8 | <0.001 *** | 0.005 (0.364) †† | 0.000 (0.678) ††† | 0.024 (0.251) † |
| O2 pulse (mL/beat/30 min) | 553.5 ± 111.2 | 651.5 ± 49.2 | 0.004 ** | 551.4 ± 129.1 | 687.7± 35.9 | 0.007 ** | 0.614 (0.014) | 0.418 (0.037) | 0.000 (0.588) ††† |
| SVi (mL/beat/30 min) | 1691.5 ± 99.4 | 1660.7 ± 102.5 | 0.018 | 1520.4 ± 117.4 | 1518.2 ± 188.1 | 0.971 | 0.007 (0.342) †† | 0.579 (0.017) | 0.629 (0.013) |
| COi (L/30 min) | 244.7 ± 20.8 | 253.7 ± 15.4 | 0.280 | 281.7 ± 22.1 | 227.2 ± 31.4 | <0.001 *** | 0.526 (0.023) | 0.002 (0.411) †† | 0.000 (0.575) ††† |
| Variables | NTG (n = 10) | p-Value | HTG (n = 10) | p-Value | p-Value (ɳ2) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Group | Time | Interaction | |||
| SDNN (ms) | 61.0 ± 4.5 | 50.1 ± 6.8 | 0.001 ** | 56.6 ± 14.4 | 66.9 ± 11.0 | 0.001 ** | 0.157 (0.108) | 0.827 (0.003) | 0.000 (0.732) ††† |
| RMSSD (ms) | 37.6 ± 7.7 | 23.7 ± 4.9 | <0.001 *** | 32.5 ± 10.4 | 44.8 ± 12.9 | 0.002 ** | 0.055 (0.190) | 0.646 (0.012) | 0.000 (0.777) ††† |
| LF band (ms2) | 7.1 ± 0.6 | 6.7 ± 0.4 | 0.001 ** | 7.0 ± 0.7 | 7.2 ± 0.6 | 0.052 | 0.399 (0.040) | 0.013 (0.294) † | 0.000 (0.616) ††† |
| HF band (ms2) | 6.5 ± 0.4 | 5.9 ± 0.3 | <0.001 *** | 6.0 ± 1.1 | 6.9 ± 1.0 | 0.003 ** | 0.455 (0.031) | 0.353 (0.048) | 0.000 (0.693) ††† |
| LF/HF band ratio | 1.2 ± 0.1 | 1.3 ± 0.2 | 0.207 | 1.5 ± 0.5 | 1.2 ± 0.3 | 0.008 ** | 0.447 (0.032) | 0.012 (0.304) † | 0.002 (0.420) †† |
| Variables | NTG (n = 10) | p-Value | HTG (n = 10) | p-Value | p-Value (ɳ2) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Group | Time | Interaction | |||
| WBC count (103/µL) | 5.4 ± 0.5 | 5.6 ± 0.6 | 0.124 | 4.6 ± 0.8 | 6.4 ± 2.1 | 0.013 * | 0.977 (0.000) | 0.003 (0.387) †† | 0.014 (0.293) † |
| Eosinophil count (%) | 3.8 ± 1.5 | 3.7 ± 1.6 | 0.623 | 3.4 ± 1.3 | 3.1 ± 1.4 | 0.153 | 0.459 (0.031) | 0.126 (0.125) | 0.286 (0.063) |
| Neutrophil count (%) | 48.5 ± 5.8 | 46.4 ± 4.8 | 0.312 | 44.6 ± 4.6 | 53.0 ± 11.1 | 0.004 ** | 0.635 (0.013) | 0.047 (0.201) † | 0.002 (0.416) †† |
| Basophil count (%) | 1.3 ± 0.5 | 1.2 ± 0.6 | 0.434 | 0.8 ± 0.1 | 0.6 ± 0.1 | <0.001 | 0.004 (0.385) †† | 0.018 (0.273) † | 0.343 (0.050) |
| Monocyte count (%) | 9.2 ± 2.6 | 9.8 ± 2.0 | 0.141 | 9.1 ± 0.7 | 7.3 ± 0.7 | <0.001 *** | 0.090 (0.151) | 0.023 (0.255) † | 0.000 (0.580) ††† |
| NK cell count (%) | 22.0 ± 4.9 | 21.3 ± 4.9 | 0.603 | 19.6 ± 5.1 | 19.0 ± 5.6 | 0.622 | 0.280 (0.064) | 0.467 (0.030) | 0.988 (0.000) |
| B cell count (%) | 14.6 ± 2.9 | 13.1 ± 3.8 | 0.048 * | 16.7 ± 2.0 | 17.6 ± 1.6 | 0.229 | 0.008 (0.331) †† | 0.548 (0.020) | 0.022 (0.258) † |
| T cell count (%) | 66.6 ± 8.0 | 65.9 ± 10.3 | 0.633 | 70.1 ± 6.9 | 71.1 ± 8.1 | 0.126 | 0.249 (0.073) | 0.780 (0.004) | 0.256 (0.071) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, W.-S.; Kim, S.-W.; Park, H.-Y. Interval Hypoxic Training Enhances Athletic Performance and Does Not Adversely Affect Immune Function in Middle- and Long-Distance Runners. Int. J. Environ. Res. Public Health 2020, 17, 1934. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17061934
Jung W-S, Kim S-W, Park H-Y. Interval Hypoxic Training Enhances Athletic Performance and Does Not Adversely Affect Immune Function in Middle- and Long-Distance Runners. International Journal of Environmental Research and Public Health. 2020; 17(6):1934. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17061934
Chicago/Turabian StyleJung, Won-Sang, Sung-Woo Kim, and Hun-Young Park. 2020. "Interval Hypoxic Training Enhances Athletic Performance and Does Not Adversely Affect Immune Function in Middle- and Long-Distance Runners" International Journal of Environmental Research and Public Health 17, no. 6: 1934. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17061934