Caenorhabditis elegans to Model the Capacity of Ascorbic Acid to Reduce Acute Nitrite Toxicity under Different Feed Conditions: Multivariate Analytics on Behavioral Imaging
Abstract
:1. Introduction
2. Material and Methods
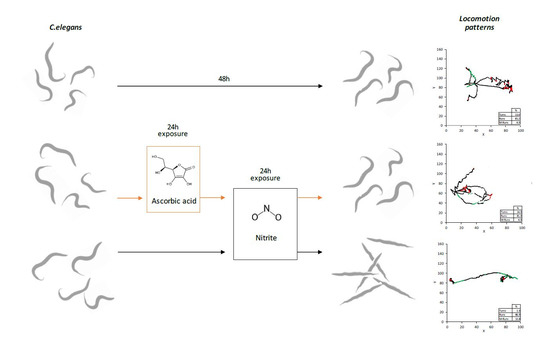
2.1. C. elegans Preparation and Exposure Protocols
2.2. Artificial Vision System and Tracking Video Capturing
2.3. Video Preprocessing
2.4. Behavioral Analysis
- M: summation of total displacement in pixels
- t: time used to complete M
- V: velocity of movement during the recorded minute, calculated from M and t
- Am: effective area of movement, calculated as
- ACT: percentage of activity. It represents the amount of nematodes with recorded movement from the total, expressed as a percentage
- M/Am: the relation between M and Am, interpreted as the amount of movement per unit of effective area
- M/ACT: the relation between M and Am, interpreted as the amount of movement per active nematode.
2.5. Statistical Analysis
3. Results
3.1. Lethality Assay
3.2. Behavior Analysis
3.2.1. Block A: Locomotion Metrics
3.2.2. Block B: Postural Dynamics
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Dodds, W.K.; Burgin, A.J.; Marcarelli, A.M.; Strauss, E.A. Nitrogen Transformations. In Methods in Stream Ecology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2017; Available online: http://0-doi-org.brum.beds.ac.uk/10.1016/B978-0-12-813047-6.00010-3 (accessed on 6 January 2017).
- Bedale, W.; Sindelar, J.J.; Milkowski, A.L. Dietary Nitrate and Nitrite: Benefits, Risks, and Evolving Perceptions. Meat Sci. 2016, 120, 85–92. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Risk assessment of nitrate and nitrite in feed. EFSA J. 2020, 18, 6290. [Google Scholar] [CrossRef]
- Chan, T.Y.K. Vegetable-Borne Nitrate and Nitrite and the Risk of Methaemoglobinaemia. Toxicol. Lett. 2011, 200, 107–108. [Google Scholar] [CrossRef]
- Tannenbaum, S.R. Preventive Action of Vitamin C on Nitrosamine Formation. Int. J. Vitam. Nutr. Res. Suppl. Int. Z. Vitam. Ernährungsforschung. Suppl. 1989, 30, 109–113. [Google Scholar]
- Tannenbaum, S.R.; Wishnok, J.S.; Leaf, C.D. Inhibition of Nitrosamine Formation by Ascorbic Acid. Am. J. Clin. Nutr. 1991, 53, 247S–250S. [Google Scholar] [CrossRef]
- Arranz, N.; Haza, A.I.; García, A.; Rafter, J.; Morales, P. Protective Effect of Vitamin C towards N-Nitrosamine-Induced DNA Damage in the Single-Cell Gel Electrophoresis (SCGE)/HepG2 Assay. Toxicol. Vitro 2007, 21, 1311–1317. [Google Scholar] [CrossRef]
- Ohsawa, K.I.; Nakagawa, S.Y.; Kimura, M.; Shimada, C.; Tsuda, S.; Kabasawa, K.; Kawaguchi, S.; Sasaki, Y.F. Detection of in Vivo Genotoxicity of Endogenously Formed N-Nitroso Compounds and Suppression by Ascorbic Acid, Teas and Fruit Juices. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2003, 539, 65–76. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Ghasemi, A.; Carlström, M.; Azizi, F.; Hadaegh, F. Vitamin C Intake Modify the Impact of Dietary Nitrite on the Incidence of Type 2 Diabetes: A 6-Year Follow-up in Tehran Lipid and Glucose Study. Nitric Oxide Biol. Chem. 2017, 62, 24–31. [Google Scholar] [CrossRef]
- Leung, M.C.K.; Williams, P.L.; Benedetto, A.; Au, C.; Helmcke, K.J.; Aschner, M.; Meyer, J.N. Caenorhabditis Elegans: An Emerging Model in Biomedical and Environmental Toxicology. Toxicol. Sci. 2008, 106, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Kaletta, T.; Hengartner, M.O. Finding Function in Novel Targets: C. Elegans as a Model Organism. Nat. Rev. Drug Discov. 2006, 5, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Shibamura, A.; Ikeda, T.; Nishikawa, Y. A Method for Oral Administration of Hydrophilic Substances to Caenorhabditis Elegans: Effects of Oral Supplementation with Antioxidants on the Nematode Lifespan. Mech. Ageing Dev. 2009, 130, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Bakaev, V.V.; Lyudmila, M.B. Effect of Ascorbic Acid on Longevity in the Nematoda Caenorhabditis Elegans. Biogerontology 2002, 3 (Suppl. 1), 23–24. [Google Scholar]
- He, F. Common Worm Media and Buffers. Bio-101 2011, e55. [Google Scholar] [CrossRef]
- Stiernagle, T. Maintenance of C. Elegans. WormBook 2006. [Google Scholar] [CrossRef] [Green Version]
- Sonane, M.; Moin, N.; Satish, A. The Role of Antioxidants in Attenuation of Caenorhabditis Elegans Lethality on Exposure to TiO2 and ZnO Nanoparticles. Chemosphere 2017, 187, 240–247. [Google Scholar] [CrossRef]
- Zhou, D.; Yang, J.; Li, H.; Cui, C.; Yu, Y.; Liu, Y.; Lin, K. The Chronic Toxicity of Bisphenol A to Caenorhabditis Elegans after Long-Term Exposure at Environmentally Relevant Concentrations. Chemosphere 2016, 154, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Höss, S.; Henschel, T.; Haitzer, M.; Traunspurger, W.; Steinberg, C.E.W. Toxicity of Cadmium to Caenorhabditis Elegans (Nematoda) in Whole Sediment and Pore Water-The Ambiguous Role of Organic Matter. Environ. Toxicol. Chem. 2001, 20, 2794–2801. [Google Scholar] [CrossRef]
- Schindelin, J.; Rueden, C.T.; Hiner, M.C.; Eliceiri, K.W. The ImageJ Ecosystem: An Open Platform for Biomedical Image Analysis. Mol. Reprod. Dev. 2015, 82, 518–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez-Marin, A.; Stephens, G.J.; Brown, A.E.X. Hierarchical Compression of Caenorhabditis Elegans Locomotion Reveals Phenotypic Differences in the Organization of Behaviour. J. R. Soc. Interface 2016, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierce-Shimomura, J.T.; Morse, T.M.; Lockery, S.R. The Fundamental Role of Pirouettes in Caenorhabditis Elegans Chemotaxis. J. Neurosci. Off. J. Soc. Neurosci. 1999, 19, 9557–9569. [Google Scholar] [CrossRef] [Green Version]
- Pierce-Shimomura, J.T. Analysis of the Effects of Turning Bias on Chemotaxis in C. Elegans. J. Exp. Biol. 2005, 208, 4727–4733. [Google Scholar] [CrossRef] [Green Version]
- Nitrate in Vegetables—Scientific Opinion of the Panel on Contaminants in the Food Chain. EFSA J. 2008, 6, 689. [CrossRef]
- Hoss, S.; Schlottmann, K.; Traunspurger, W. Toxicity of Ingested Cadmium to the Nematode Caenorhabditis Elegans. Environ. Sci. Technol. 2011, 45, 10219–10225. [Google Scholar] [CrossRef]
- Avery, L.; You, Y.-J. C. Elegans Feeding. In The C. elegans Research Community; WormBook: Pasadena, CA, USA, 2012. [Google Scholar] [CrossRef]
- Li, J.; Li, D.; Yang, Y.; Xu, T.; Li, P.; He, D. Acrylamide Induces Locomotor Defects and Degeneration of Dopamine Neurons in Caenorhabditis Elegans. J. Appl. Toxicol. 2016, 36, 60–67. [Google Scholar] [CrossRef]
- Özen, H.; Kamber, U.; Karaman, M.; Gül, S.; Atakişi, E.; Özcan, K.; Atakişi, O. Histopathologic, Biochemical and Genotoxic Investigations on Chronic Sodium Nitrite Toxicity in Mice. Exp. Toxicol. Pathol. 2014, 66, 367–375. [Google Scholar] [CrossRef]
- De Baere, I.; Liu, L.; Moens, L.; Van Beeumen, J.; Gielens, C.; Richelle, J.; Trotman, C.; Finch, J.; Gerstein, M.; Perutz, M. Polar Zipper Sequence in the High-Affinity Hemoglobin of Ascaris Suum: Amino Acid Sequence and Structural Interpretation. Proc. Natl. Acad. Sci. USA 1992, 89, 4638–4642. [Google Scholar] [CrossRef] [Green Version]
- Minning, D.M.; Gow, A.J.; Bonavetura, J.; Braun, R.; Dewhirst, M.; Goldberg, D.E.; Stamler, J.S. Ascaris Haemoglobin Is a Nitric Oxide-Activated “Deoxygenase”. Nature 1999, 401, 497–502. [Google Scholar] [CrossRef]
- Carrillo, M.A.; Guillermin, M.L.; Rengarajan, S.; Okubo, R.P.; Hallem, E.A. O2-Sensing Neurons Control CO2 Response in C. Elegans. J. Neurosci. 2013, 33, 9675–9683. [Google Scholar] [CrossRef]
- Park, E.C.; Rongo, C. The P38 MAP Kinase Pathway Modulates the Hypoxia Response and Glutamate Receptor Trafficking in Aging Neurons. eLife 2016, 5, 3–5. [Google Scholar] [CrossRef]
- Vermeer, I.T.M.; Engels, L.G.J.B.; Pachen, D.M.F.A.; Dallinga, J.W.; Kleinjans, J.C.S.; Van Maanen, J.M.S. Intragastric Volatile N-Nitrosamines, Nitrite, PH, and Helicobacter Pylori during Long-Term Treatment with Omeprazole. Gastroenterology 2001, 121, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Sarkis, G.J.; Kurpiewski, M.R.; Ashcom, J.D.; Jen-Jacobson, L.; Jacobson, L.A. Proteases of the Nematode Caenorhabditis Elegans. Arch. Biochem. Biophys. 1988, 261, 80–90. [Google Scholar] [CrossRef]
- Hanafy, K.A.; Krumenacker, J.S.; Murad, F. NO, Nitrotyrosine, and Cyclic GMP in Signal Transduction. Med. Sci. Monit 2001, 7, 801–819. [Google Scholar]
- Marshall, H.E. Nitrosation and Oxidation in the Regulation of Gene Expression. FASEB J. 2000, 14, 1889–1900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gusarov, I.; Gautier, L.; Smolentseva, O.; Shamovsky, I.; Eremina, S.; Mironov, A.; Nudler, E. Bacterial Nitric Oxide Extends the Lifespan of C. Elegans. Cell 2013, 152, 818–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Descriptors of Block-A | R2 |
|---|---|
| M | 0.93 |
| t | 0.91 |
| V | 0.92 |
| Am | 0.91 |
| ACT | 0.92 |
| M/Am | 0.86 |
| M/ACT | 0.89 |
| Mortality | 0.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verdu, S.; Perez, A.J.; Carrascosa, C.; Barat, J.M.; Talens, P.; Grau, R. Caenorhabditis elegans to Model the Capacity of Ascorbic Acid to Reduce Acute Nitrite Toxicity under Different Feed Conditions: Multivariate Analytics on Behavioral Imaging. Int. J. Environ. Res. Public Health 2021, 18, 2068. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18042068
Verdu S, Perez AJ, Carrascosa C, Barat JM, Talens P, Grau R. Caenorhabditis elegans to Model the Capacity of Ascorbic Acid to Reduce Acute Nitrite Toxicity under Different Feed Conditions: Multivariate Analytics on Behavioral Imaging. International Journal of Environmental Research and Public Health. 2021; 18(4):2068. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18042068
Chicago/Turabian StyleVerdu, Samuel, Alberto J. Perez, Conrado Carrascosa, José M. Barat, Pau Talens, and Raúl Grau. 2021. "Caenorhabditis elegans to Model the Capacity of Ascorbic Acid to Reduce Acute Nitrite Toxicity under Different Feed Conditions: Multivariate Analytics on Behavioral Imaging" International Journal of Environmental Research and Public Health 18, no. 4: 2068. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18042068