A Review of Evidence-Based Recommendations for Pericoronitis Management and a Systematic Review of Antibiotic Prescribing for Pericoronitis among Dentists: Inappropriate Pericoronitis Treatment Is a Critical Factor of Antibiotic Overuse in Dentistry
Abstract
:1. Introduction
- Enhancing infection prevention and control;
- Controlling source control;
- Prescribing antibiotics when they are truly needed;
- Prescribing appropriate antibiotics;
- Prescribing antibiotics with appropriate dosage;
- Reassessing treatment when culture results are available;
- Using the shortest duration of antibiotics based on evidence;
- Educating staff;
- Supporting surveillance of antimicrobial resistance and healthcare-associated infections and monitoring of antibiotic consumption;
- Supporting an interdisciplinary approach.
- (a)
- Provide the most recent, clinically significant, and evidence-based recommendations for pericoronitis diagnosis and proper treatment (Part A);
- (b)
- Systematically review antibiotic prescribing for pericoronitis (Part B).
2. Part A: Pericoronitis Evidence-Based Therapy
2.1. Methods
2.2. Classification
- Transient—occurs during the tooth eruption;
- Non-transient—occurs after the tooth eruption is terminated.
- Acute—sudden onset, severe symptoms;
- Chronic—protracted, mild or no symptoms.
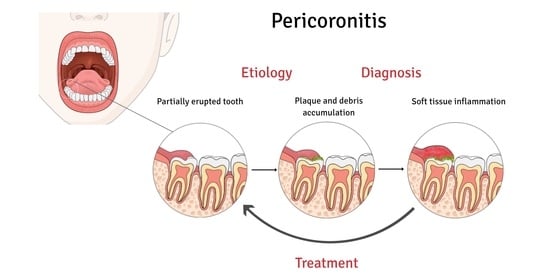
2.3. Etiopathogenesis
- There is no significant difference in the chance of pericoronitis between class I and II of Pell and Gregory classification;
- Third molars classified in position A had a greater chance of pericoronitis when compared to those in position B of Pell and Gregory classification;
- The vertical position of the lower third molar (Winter’s classification) is more associated with the occurrence of pericoronitis when compared to the other positions, while the horizontal position decreases the occurrence of pericoronitis [29].
2.4. Clinical Manifestation and Diagnosis
2.5. Complications
2.6. Therapy
2.6.1. Infection Management
- Local intervention [47]
- Irrigation of pericoronal space with a sterile solution (aqua pro injectione, saline, antiseptics for mucosa, e.g., hydrogen peroxide or chlorhexidine).
- Mechanical removal of plaque and debris (debridement) from the pocket using periodontal instruments and swabs gently.
- Irrigation and debridement may be combined to achieve better results.
- Any collection of pus should be drained.
- Traumatic occlusion, if present, should be prevented by soft tissue or occlusal adjustment. Extraction of antagonist tooth may be considered.
- The patient should be instructed in oral hygiene involving gentle and careful mechanical cleaning of the affected area and mouth rinsing with antiseptics (e.g., 0.12–0.2% chlorhexidine two times daily for 1 min).
- B.
- Antibiotics
- C.
- Photodynamic therapy
- D.
- Follow-up to check the effectiveness of treatment
2.6.2. Pain Management
- A.
- Oral analgesics
- B.
- Topical analgesics
2.6.3. Prevention
- A.
- Tooth extraction
Specific attention is drawn to plaque formation and pericoronitis. Plaque formation is a risk factor but is not in itself an indication for surgery. The degree to which the severity or recurrence rate of pericoronitis should influence the decision for surgical removal of a third molar remains unclear. The evidence suggests that a first episode of pericoronitis, unless particularly severe, should not be considered an indication for surgery. Second or subsequent episodes should be considered the appropriate indication for surgery.
- B.
- Pericoronal tissue surgery
- C.
- Oral hygiene
2.7. Discussion
- The diagnosis of pericoronitis is late due to failure to seek a medical examination or poor diagnosis. This contributes to the development of infection spread and systemic symptoms.
- Improper treatment of pericoronitis can worsen the patient’s condition due to the treatment itself and delaying the proper care.
- Ignorance of principles for appropriate antibiotic therapy.
2.8. Conclusions
3. Part B: Systematic Review of Antibiotic Prescribing for Pericoronitis
3.1. Methods
3.1.1. Eligibility
3.1.2. Search Strategy
3.1.3. Data Extraction
3.2. Results
3.2.1. Study Selection
3.2.2. Study Characteristics
3.2.3. Questionnaires among Dentists
3.2.4. Studies Involving Patients Treated for Pericoronitis
3.3. Discussion
3.4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Douglass, A.B.; Douglass, J.M. Common dental emergencies. Am. Fam. Physician 2003, 67, 511–516. [Google Scholar] [PubMed]
- Nitzan, D.; Tal, O.; Sela, M.; Shteyer, A. Pericoronitis: A reappraisal of its clinical and microbiologic aspects. J. Oral Maxillofac. Surg. 1985, 43, 510–516. [Google Scholar] [CrossRef]
- Katsarou, T.; Kapsalas, A.; Souliou, C.; Stefaniotis, T.; Kalyvas, D. Pericoronitis: A clinical and epidemiological study in greek military recruits. J. Clin. Exp. Dent. 2019, 11, e133–e137. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.W. The prophylactic extraction of third molars: A public health hazard. Am. J. Public Health 2007, 97, 1554–1559. [Google Scholar] [CrossRef] [PubMed]
- Kay, L. Investigations into the nature of pericoronitis. Br. J. Oral Surg. 1966, 3, 188–205. [Google Scholar] [CrossRef]
- McNutt, M.; Partrick, M.; Shugars, D.A.; Phillips, C.; White, R.P. Impact of symptomatic pericoronitis on health-related quality of life. J. Oral Maxillofac. Surg. 2008, 66, 2482–2487. [Google Scholar] [CrossRef] [PubMed]
- Bataineh, A.B.; Al, Q.M. The predisposing factors of pericoronitis of mandibular third molars in a Jordanian population. Quintessence Int. 2003, 34, 227–231. [Google Scholar]
- Singh, P.; Nath, P.; Bindra, S.; Rao, S.S.; Reddy, K.V.R. The predictivity of mandibular third molar position as a risk indicator for pericoronitis: A prospective study. Natl. J. Maxillofac. Surg. 2018, 9, 215–221. [Google Scholar] [CrossRef]
- Moloney, J.; Stassen, L. Pericoronitis: Treatment and a clinical dilemma. J. Ir. Dent. Assoc. 2009, 55, 190–192. [Google Scholar]
- Wehr, C.; Cruz, G.; Young, S.; Fakhouri, W.D. An insight into acute pericoronitis and the need for an evidence-based standard of care. J. Dent. 2019, 7, 88. [Google Scholar] [CrossRef] [Green Version]
- The Global Alliance for Infections in Surgery. 10 Principles for an Appropriate Antibiotic Therapy in Surgery. 2019. Available online: https://infectionsinsurgery.org/principles-of-antibiotic-therapy-in-surgery-3/ (accessed on 5 September 2020).
- Hicks, L.A.; Bartoces, M.G.; Roberts, R.M.; Suda, K.J.; Hunkler, R.J.; Taylor, T.H., Jr.; Schrag, S.J. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2015, 60, 1308–1316. [Google Scholar] [CrossRef] [Green Version]
- CDC. Outpatient Antibiotic Prescriptions—United States, 2016, 2018; Centers for Disease Control Prevention: Atlanta, GA, USA, 2019. [Google Scholar]
- Demirjian, A.; Sanchez, G.V.; Finkelstein, J.A.; Ling, S.M.; Srinivasan, A.; Pollack, L.A.; Hicks, L.A.; Iskander, J.K. CDC Grand rounds: Getting smart about antibiotics. Mmwr. Morb. Mortal. Wkly. Rep. 2015, 64, 871–873. [Google Scholar] [CrossRef] [Green Version]
- Koyuncuoglu, C.Z.; Aydin, M.; Kirmizi, N.I.; Aydin, V.; Aksoy, M.; Isli, F.; Akici, A. Rational use of medicine in dentistry: Do dentists prescribe antibiotics in appropriate indications? Eur. J. Clin. Pharm. 2017, 73, 1027–1032. [Google Scholar] [CrossRef]
- Marra, F.; George, D.; Chong, M.; Sutherland, S.; Patrick, D.M. Antibiotic prescribing by dentists has increased: Why? J. Am. Dent. Assoc. 2016, 147, 320–327. [Google Scholar] [CrossRef] [Green Version]
- Baudet, A.; Kichenbrand, C.; Pulcini, C.; Descroix, V.; Lesclous, P.; Thilly, N.; Clément, C.; Guillet, J. Antibiotic use and resistance: A nationwide questionnaire survey among French dentists. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1295–1303. [Google Scholar] [CrossRef]
- Combes, J.; Pepper, T.; Bryce, G.; MacBeth, N. Dental care provision to UK military personnel serving on Operation Herrick in Afghanistan. Part 2: Aetiology and management. Br. Dent. J. 2019, 226, 50–54. [Google Scholar] [CrossRef]
- Bjelovucic, R.; Par, M.; Rubcic, D.; Marovic, D.; Prskalo, K.; Tarle, Z. Antibiotic prescription in emergency dental service in Zagreb, Croatia—A retrospective cohort study. Int. Dent. J. 2019, 69, 273–280. [Google Scholar] [CrossRef]
- Salako, N.O.; Rotimi, V.O.; Adib, S.M.; Al-Mutawa, S. Pattern of antibiotic prescription in the management of oral diseases among dentists in Kuwait. J. Dent. 2004, 32, 503–509. [Google Scholar] [CrossRef]
- Palmer, N.A.O.; Pealing, R.; Ireland, R.S.; Martin, M.V. A study of therapeutic antibiotic prescribing in National Health Service general dental practice in England. Br. Dent. J. 2000, 188, 554–558. [Google Scholar] [CrossRef] [Green Version]
- Sixou, J.-L.; Magaud, C.; Jolivet-Gougeon, A.; Cormier, M.; Bonnaure-Mallet, M. Evaluation of the mandibular third molar pericoronitis flora and its susceptibility to different antibiotics prescribed in France. J. Clin. Microbiol. 2003, 41, 5794–5797. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Pérez, J.L. Third molar infections. Med. Oral Patol. Oral Cir. Bucal 2004, 9, 122–125. [Google Scholar]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef] [Green Version]
- Hazza’a, A.M.; Bataineh, A.B.; Odat, A.A. Angulation of mandibular third molars as a predictive factor for pericoronitis. J. Contemp. Dent. Pr. 2009, 10, 51–58. [Google Scholar]
- Primo, F.T.; Primo, B.T.; Scheffer, M.A.R.; Hernández, P.A.G.; Rivaldo, E.G. Evaluation of 1211 third molars positions according to the classification of winter, Pell & Gregory. Int. J. Odontostomat. 2017, 11, 61–65. [Google Scholar]
- Tsvetanov, T. Association of the mandibular third molar position to the pericoronitis. Int. J. Med. Res. Health Sci. 2018, 7, 35–40. [Google Scholar]
- McArdle, L.W.; Andiappan, M.; Khan, I.; Jones, J.; McDonald, F. Diseases associated with mandibular third molar teeth. Br. Dent. J. 2018, 224, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Galvão, E.L.; da Silveira, E.M.; de Oliveira, E.S.; da Cruz, T.M.M.; Flecha, O.D.; Falci, S.G.M.; Gonçalves, P.F. Association between mandibular third molar position and the occurrence of pericoronitis: A systematic review and meta-analysis. Arch. Oral Biol. 2019, 107, 104486. [Google Scholar] [CrossRef] [PubMed]
- Meurman, J.H.; Rajasuo, A.; Murtomaa, H.; Savolainen, S. Respiratory tract infections and concomitant pericoronitis of the wisdom teeth. BMJ 1995, 310, 834–836. [Google Scholar] [CrossRef] [Green Version]
- Dhabhar, F.S. Effects of stress on immune function: The good, the bad, and the beautiful. Immunol. Res. 2014, 58, 193–210. [Google Scholar] [CrossRef]
- Oertelt-Prigione, S. Immunology and the menstrual cycle. Autoimmun. Rev. 2012, 11, A486–A492. [Google Scholar] [CrossRef]
- Oguzulgen, I.K.; Turktas, H.; Erbas, D. Airway inflammation in premenstrual asthma. J. Asthma 2002, 39, 517–522. [Google Scholar] [CrossRef]
- Pooja Umaiyal, M.; Ramamurthy, J. Prevalence of mandibular third molar pericoronitis among smokers and evaluation of its treatment outcomes—A retrospective study. Int. J. Pharm. Sci. Res. 2020, 11, 452–458. [Google Scholar] [CrossRef]
- Tripodi, D.; Cosi, A.; Fulco, D.; D’Ercole, S. The impact of sport training on oral health in athletes. J. Dent. 2021, 9, 51. [Google Scholar] [CrossRef]
- Needleman, I.; Ashley, P.; Fine, P.; Haddad, F.; Loosemore, M.; de Medici, A.; Donos, N.; Newton, T.; van Someren, K.; Moazzez, R.; et al. Oral health and elite sport performance. Br. J. Sports. Med. 2015, 49, 3–6. [Google Scholar] [CrossRef]
- Ashley, P.; Di Iorio, A.; Cole, E.; Tanday, A.; Needleman, I. Oral health of elite athletes and association with performance: A systematic review. Br. J. Sports Med. 2015, 49, 14–19. [Google Scholar] [CrossRef]
- Neville, B.W.; Damm, D.D.; Allen, C.M.; Chi, A.C. Periodontal diseases. In Oral and Maxillofacial Pathology, 4th ed.; Elsevier, Inc.: St. Louis, MO, USA, 2016; p. 156. [Google Scholar]
- Yurttutan, M.; Karaahmetoğlu, Ö.; Üçok, C.; Bağış, N. Comparison of the quality of life of patients with mandibular third molars and mild pericoronitis treated by extraction or by a periodontal approach. Br. J. Oral Maxillofac. Surg. 2020, 58, 179–184. [Google Scholar] [CrossRef]
- Wray, D.; Clark, A.J. Spread and control of infection. In Textbook of General and Oral Surgery; Churchill Livingstone: London, UK, 2003. [Google Scholar]
- Dhonge, R.P.; Zade, R.; Gopinath, V.; Amirisetty, R. An insight into pericoronitis. Int. J. Dent. Med. Res. 2015, 1, 172–175. [Google Scholar]
- Basson, O.; Kilborn, T. Tonsillar Abscess (Quinsy). In ABC of Pediatric Surgical Imaging; Andronikou, S., Alexander, A., Kilborn, T., Millar, A.J.W., Daneman, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 146–147. [Google Scholar] [CrossRef]
- Galioto, N.J. Peritonsillar abscess. Am. Fam. Physician 2017, 95, 501–506. [Google Scholar]
- Candamourty, R.; Venkatachalam, S.; Babu, M.R.; Kumar, G.S. Ludwig’s Angina—An emergency: A case report with literature review. J. Nat. Sci. Biol. Med. 2012, 3, 206. [Google Scholar] [CrossRef] [Green Version]
- Chou, Y.-K.; Lee, C.-Y.; Chao, H.-H. An upper airway obstruction emergency: Ludwig angina. Pediatr. Emerg. Care 2007, 23, 892–896. [Google Scholar] [CrossRef] [Green Version]
- Campbell, J.H. Pathology associated with the third molar. Oral. Maxillofac. Surg. Clin. North. Am. 2013, 25, 1–10. [Google Scholar] [CrossRef]
- Avery, B.; Brown, J.; Carter, J.; Corrigan, A.; Haskell, R.; Leopard, P.; Williams, J.; Loukota, R.; Lowry, J.; McManners, J. Management of pericoronitis. In National Clinical Guidelines 1997, 2nd ed.; Gregg, T.A., Ed.; The Faculty of Dental Surgery of the Royal College of Surgeons of England: London, UK, 2004; pp. 19–21. [Google Scholar]
- Johri, A.; Piecuch, J.F. Should teeth be extracted immediately in the presence of acute infection. Oral Maxillofac. Surg. Clin. North. Am. 2012, 23, 507–511. [Google Scholar] [CrossRef]
- Asok, A.; Bhandary, R.; Shetty, M.; Shetty, S. Comparative evaluation of pain response in operculectomy procedures using conventional, electrocautery and Laser techniques. Manipal J. Dent. Sci. 2018, 3, 9–13. [Google Scholar]
- Ahad, A.; Tandon, S.; Lamba, A.K.; Faraz, F.; Anand, P.; Aleem, A. Diode laser assisted excision and low level laser therapy in the management of mucus extravasation cysts: A case series. J. Lasers Med. Sci. 2017, 8, 155. [Google Scholar] [CrossRef] [Green Version]
- Aulestia-Viera, P.V.; Braga, M.M.; Borsatti, M.A. The effect of adjusting the pH of local anaesthetics in dentistry: A systematic review and meta-analysis. Int. Endod. J. 2018, 50, 862–876. [Google Scholar] [CrossRef] [Green Version]
- Joint Formulary Committee. BNF 80 (British National Formulary) September 2020; Pharmaceutical Press: London, UK, 2020. [Google Scholar]
- Palmer, N.; Longman, L.; Randall, C.; Pankhurst, C. Pericoronitis. In Antimicrobial Prescribing for General Dental Practitioners, 3rd ed.; Palmer, N., Ed.; Faculty of General Dental Practice and Faculty of Dental Surgery: London, UK, 2020. [Google Scholar]
- Martins, J.R.; Chagas, O.L., Jr.; Velasques, B.D.; Bobrowski, Â.N.; Correa, M.B.; Torriani, M.A. The use of antibiotics in odontogenic infections: What is the best choice? A systematic review. J. Oral. Maxillofac. Surg. 2017, 75, 2606.e2601–2606.e2611. [Google Scholar] [CrossRef]
- Aoun, G.; Yared, G.; Diab, H.A.; Berberi, A. Antibiotic therapy and bacterial odontogenic infections: An overview. World J. Dent. 2018, 9, 154–161. [Google Scholar] [CrossRef]
- Matthews, D.C.; Sutherland, S.; Basrani, B. Emergency management of acute apical abscesses in the permanent dentition: A systematic review of the literature. J. Can. Dent. Assoc. 2003, 69, 660. [Google Scholar]
- Liu, Y.; Qin, R.; Zaat, S.A.J.; Breukink, E.; Heger, M. Antibacterial photodynamic therapy: Overview of a promising approach to fight antibiotic-resistant bacterial infections. J. Clin. Transl. Res. 2015, 1, 140–167. [Google Scholar]
- Akram, Z.; Raffat, M.A.; Saad Shafqat, S.; Mirza, S.; Ikram, S. Clinical efficacy of photodynamic therapy as an adjunct to scaling and root planing in the treatment of chronic periodontitis among cigarette smokers: A systematic review and meta-analysis. Photodiagn. Photodyn. 2019, 26, 334–341. [Google Scholar] [CrossRef]
- Gursoy, H.; Ozcakir-Tomruk, C.; Tanalp, J.; Yılmaz, S. Photodynamic therapy in dentistry: A literature review. Clin. Oral Investig. 2013, 17, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, I.J.; Dougherty, T.J. Basic principles of photodynamic therapy. J. Porphyr. Phthalocyanines. 2001, 5, 105–129. [Google Scholar] [CrossRef]
- Elsadek, M.F.; Ahmed, B.M.; Eskandrani, R.M. Level of pain intensity, cytokine profiling and microbial load after photodynamic therapy in acute severe pericoronitis. Photodiagn. Photodyn. 2020, 31, 101830. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Mukhtar, H. Mechanism of photodynamic therapy-induced cell death. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2000; Volume 319, pp. 342–358. [Google Scholar] [CrossRef]
- Corrêa, M.G.; Oliveira, D.H.; Saraceni, C.H.C.; Ribeiro, F.V.; Pimentel, S.P.; Cirano, F.R.; Casarin, R.C.V. Short-term microbiological effects of photodynamic therapy in non-surgical periodontal treatment of residual pockets: A split-mouth RCT. Lasers Surg. Med. 2016, 48, 944–950. [Google Scholar] [CrossRef]
- Eroglu, C.N.; Keskin Tunc, S.; Erten, R.; Usumez, A. Clinical and histological evaluation of the efficacy of antimicrobial photodynamic therapy used in addition to antibiotic therapy in pericoronitis treatment. Photodiagn. Photodyn. 2018, 21, 416–420. [Google Scholar] [CrossRef]
- Magraw, C.B.; Golden, B.; Phillips, C.; Tang, D.T.; Munson, J.; Nelson, B.P.; White, R.P., Jr. Pain with pericoronitis affects quality of life. J. Oral Maxillofac. Surg. 2015, 73, 7–12. [Google Scholar] [CrossRef]
- Alalwani, A.; Buhara, O.; Tüzüm, M.Ş. Oral Health-related quality of life and the use of oral and topical nonsteroidal anti-inflammatory drugs for pericoronitis. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2019, 25, 9200. [Google Scholar] [CrossRef]
- SDCEP. Drug Prescribing for Dentistry: Scottish Dental Clinical Effectiveness Programme, 3rd ed.; SDCEP: Dundee, UK, 2016; pp. 52–53. [Google Scholar]
- De Freiras, G.C.; Pozzobon, R.T.; Blaya, D.S.; Moreira, C.H. Efficacy of benzocaine 20% topical anesthetic compared to placebo prior to administration of local anesthesia in the oral cavity: A randomized controlled trial. Anesth. Prog. 2015, 62, 46–50. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-S. Recent advances in topical anesthesia. J. Dent. Anesth. Pain Med. 2016, 16, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Franz-Montan, M.; Ribeiro, L.N.d.M.; Volpato, M.C.; Cereda, C.M.S.; Groppo, F.C.; Tofoli, G.R.; de Araújo, D.R.; Santi, P.; Padula, C.; de Paula, E. Recent advances and perspectives in topical oral anesthesia. Expert Opin. Drug. Deliv. 2017, 14, 673–684. [Google Scholar] [CrossRef] [Green Version]
- Svensson, P.; Bjerring, P.; Arendt-Nielsen, L.; Kaaber, S. Hypoalgesic effect of EMLA and lidocaine gel applied on human oral mucosa: Quantitative evaluation by sensory and pain thresholds to argon laser stimulation. Anesth. Prog. 1992, 39, 4–8. [Google Scholar]
- Friskopp, J.; Nilsson, M.; Isacsson, G. The anesthetic onset and duration of a new lidocaine/prilocaine gel intra-pocket anesthetic (Oraqix®) for periodontal scaling/root planing. J. Clin. Periodontol. 2001, 28, 453–458. [Google Scholar] [CrossRef]
- Newman, M.G.; Takei, H.; Klokkevold, P.R.; Carranza, F.A. Chapter 42 periodontal treatment for older adults. In Newman and Carranza’s Clinical Periodontology E-Book; Sue, S., Spackman, J.G.B., Eds.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018; p. 2598. [Google Scholar]
- Borzabadi-Farahani, A.; Cronshaw, M. Lasers in orthodontics. In Lasers in Dentistry—Current Concepts; Coluzzi, D., Parker, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 252–253. [Google Scholar] [CrossRef]
- Borzabadi-Farahani, A. The adjunctive soft-tissue diode laser in orthodontics. Compend. Contin. Educ. Dent. 2017, 38, e18–e31. [Google Scholar]
- Aoki, A.; Mizutani, K.; Schwarz, F.; Sculean, A.; Yukna, R.A.; Takasaki, A.A.; Romanos, G.E.; Taniguchi, Y.; Sasaki, K.M.; Zeredo, J.L.; et al. Periodontal and peri-implant wound healing following laser therapy. Periodontol. 2000 2015, 68, 217–269. [Google Scholar] [CrossRef]
- Balamurugan, R.; Benson, N. Comparison of two treatment modalities: Operculectomy vs. third molar removal for management of pericoronitis. J. Dent. Res. Pr. 2019, 1, 7–10. [Google Scholar]
- Caymaz, M.G.; Buhara, O. Association of oral hygiene and periodontal health with third molar pericoronitis: A cross-sectional study. Biomed. Res. Int. 2021, 2021, 6664434. [Google Scholar] [CrossRef]
- Ramadan, A.M.; Rikaby, O.A.A.; Abu-Hammad, O.A.; Dar-Odeh, N.S. Knowledge and attitudes towards antibiotic prescribing among dentists in Sudan. Pesqui. Bras. Odontopediatr. Clín. Integr. 2019, 19. [Google Scholar] [CrossRef]
- Vessal, G.; Khabiri, A.; Mirkhani, H.; Cookson, B.D.; Askarian, M. Study of antibiotic prescribing among dental practitioners in Shiraz, Islamic Republic of Iran. East. Mediterr. Health J. 2011, 17, 763–769. [Google Scholar] [CrossRef]
- Mahmoodi, B.; Weusmann, J.; Azaripour, A.; Braun, B.; Walter, C.; Willershausen, B. Odontogenic infections: A 1-year retrospective study. J. Contemp. Dent. Pr. 2015, 16, 253–258. [Google Scholar] [CrossRef]
- Cope, A.L.; Francis, N.A.; Wood, F.; Chestnutt, I.G. Antibiotic prescribing in UK general dental practice: A cross-sectional study. Commun. Dent. Oral Epidemiol. 2016, 44, 145–153. [Google Scholar] [CrossRef]
- Tulip, D.E.; Palmer, N.O.A. A retrospective investigation of the clinical management of patients attending an out of hours dental clinic in Merseyside under the new NHS dental contract. Br. Dent. J. 2008, 205, 659–664. [Google Scholar] [CrossRef] [Green Version]
- Nolte, O. Antimicrobial resistance in the 21st century: A multifaceted challenge. Protein. Pept. Lett. 2014, 21, 330–335. [Google Scholar] [CrossRef]
- Hoffman, P.S. Antibacterial discovery: 21st century challenges. Antibiotics 2020, 9, 213. [Google Scholar] [CrossRef]
- Ahmadi, H.; Ebrahimi, A.; Ahmadi, F. Antibiotic therapy in dentistry. Int. J. Dent. 2021, 2021, 6667624. [Google Scholar] [CrossRef]





| Term | Meaning | Etymology | Semantic Relation |
|---|---|---|---|
| Operculitis | inflammation of operculum; operculum is a clinical term for the soft tissue covering a partially erupted tooth | Latin verb operire ‘to cover’ | Hyponym of pericoronitis |
| Pericoronitis | inflammation of the tissues around the tooth crown | Greek prefix peri- ‘around’ Latin noun corona ‘crown’ Greek suffix -itis ‘inflammation of a tissue’ | Hypernym of operculitis Hyponym of dentitio difficilis |
| Dentitio difficilis | difficult teething | Latin verb dentitio ‘teethe’ Latin adjective difficilis ‘difficult’ | Hypernym of pericoronitis |
| Causes | Risk Factors | |
|---|---|---|
| Local | Systemic | |
| Pericoronitis in anamnesis | Upper respiratory tract infection | |
| Imperfectly erupted tooth | Poor oral hygiene and plaque retention | Mental or physical stress |
| Bacterial accumulation | Traumatization of pericoronal soft tissues | Diseases impairing the immune system or wound healing (diabetes mellitus) |
| Debris entrapment | Premenstrual phase | |
| Smoking | ||
| Metronidazole | |||
| Adults | Children (over 10 years) | ||
| Orally | 400 mg * | 200–250 mg * | |
| Intravenously | 500 mg ** | 7.5 mg/kg *** | |
| Notes: | * | three times daily for up to five days | |
| ** | every 8 h given over 20 min | ||
| *** | every 8 h (max. 500 mg per dose) | ||
| Amoxicillin | |||
| Adults | Children (over 12 years) | ||
| Orally | 500 mg * | 500 mg *** | |
| Intravenously | 500 mg ** | – | |
| Notes: | * | every 8 h for up to five days; 1 g every 8 h in severe infection | |
| ** | every 8 h; 1 g every 6 h in severe infection | ||
| *** | every 8 h; 1 g every 8 h in severe infection | ||
| Ibuprofen | ||
| Adults | Children | |
| 400 mg | 6–11 months | 50 mg |
| 1–3 years | 100 mg | |
| 4–6 years | 150 mg | |
| 7–9 years | 200 mg | |
| 10–11 years | 300 mg | |
| 12–17 years | 300–400 mg | |
| Notes: | The doses can be used four times a day for up to five days In adults, the dose can be increased to a maximum of 2.4 g daily | |
| Administration preferably after food | ||
| Aspirin | ||
| Adults | Children | |
| 600 mg | <16 years | – * |
| >16 years | as for adults | |
| Notes: | The doses can be used four times a day for up to five days Blood thinner Aspirin should not be prescribed after or before surgery | |
| Administration preferably after food * not recommended for children due to Reye’s syndrome | ||
| Diclofenac | ||
| Adults | Children | |
| 50 mg | – * | |
| Notes: | The doses can be used three times a day for up to five days The maximal daily dose is 150 mg * not recommended for dental use in children | |
| Topical Analgesics | Availability | Concentration | Onset Time (min) | Duration (min) | |
|---|---|---|---|---|---|
| Benzocaine * [68,69] | gel, spray, ointment, solution | 1–20% | 0.5 | 5–15 | |
| Tetracaine Hydrochloride ** [69] | spray, ointment, solution | 0.2–2.0% | 2 | 20–60 | |
| Lidocaine [69,70] | gel, spray, ointment, solution | 2–5% | 1–2 | 15 | |
| Cetacaine [69] | solution | 14% benzocaine | 0.5 | 30–60 | |
| 2% butamben | |||||
| 2% tetracaine- | |||||
| hydrochloric acid | |||||
| EMLA *** [69,70,71] | cream | 1:1 mixture of | 2 | 10 | |
| 2.5% prilocaine and 2.5% lidocaine | |||||
| Oraqix [69,72] | gel | 2.5% lidocaine and 2.5% prilocaine | 0.5 | 20 | |
| Notes: | * | risks: cross allergies to PABA and ester-type anesthetics; methemoglobinemia | |||
| ** | quickly absorbed into the mucosa, dose limitation is 20 mg per session in healthy adults | ||||
| *** | eutectic mixture of local anesthetics | ||||
| Author | Year | Country | Question Design | Number of Respondents (n) | Outcome | (n)/(On) in % | |
|---|---|---|---|---|---|---|---|
| Specification | Number (On) | ||||||
| Baudet | 2020 | France | Situation (pericoronitis) in which antibiotics were reported to be prescribed to a healthy patient. | 408 | out of (n) | 239 | 58.6 |
| Wehr | 2019 | Texas, USA | An emergency treatment preferred for acute pericoronitis involved antibiotics. | 72 | out of (n) | 41 | 56.9 |
| Ramadan | 2019 | Sudan | Pericoronitis is an indication for antibiotic prescribing. | 100 | yes | 77 | 77.0 |
| Vessal | 2011 | Iran | Dental practitioners that would prescribe antibiotics for pericoronitis. | 219 | ouf of (n) | 147 | 67.1 |
| Salako | 2004 | Kuwait | Should antibiotics be prescribed for pericoronitis? | 168 | yes | 122 | 72.6 |
| Palmer | 2000 | UK | Dental practitioners prescribing antibiotics for pericoronitis. | 929 | out of (n) | 780 | 84.0 |
| Total | 1896 | 1406 | 74.2 | ||||
| Author | Country | The Position of Pericoronitis in the Frequency of Antibiotic Use for Its Treatment within the Surveyed Diagnoses and Situations (n) | (n) |
|---|---|---|---|
| Baudet | France | 3rd | 5 |
| Wehr | Texas, USA | not specified | not specified |
| Ramadan | Sudan | 4nd | 9 |
| Vessal | Iran | 3rd | 10 |
| Salako | Kuwait | 3rd | 8 |
| Palmer | UK | 2nd | 15 |
| Author | Country | Frequency of Prescribed Antibiotics | % |
|---|---|---|---|
| Baudet ** | France | amoxicillin spiramycin + metronidazole combination amoxicillin-clavulanic acid | 65.8 11.6 10.3 |
| Wehr | Texas, USA | not specified | |
| Ramadan ** | Sudan | metronidazole amoxicillin amoxicillin-clavulanic acid | 35.0 31.4 17.4 |
| Vessal | Iran | not specified | |
| Salako * | Kuwait | amoxicillin metronidazole Penicillin | 68.7 13.0 10.4 |
| Palmer * | UK | metronidazole amoxicillin penicillin | 67 30 10 |
| Note: | * | Data for pericoronitis-related prescription | |
| ** | Data for all dental-related prescription, including pericoronitis | ||
| Author | Authors’ General Evaluation of Treatments Reported by Respondents | Need for Further Education |
|---|---|---|
| Baudet | This nationwide study… shows the same trend as in other countries in terms of high prevalence of misuse and overuse of antibiotics. | Yes |
| Wehr | Skewed reasoning for treating pericoronitis. | Yes |
| Ramadan | Shortfalls in the knowledge of the participants regarding clinical indications and choice of antibiotic. | Yes |
| Vessal | Unfortunately, more than 60% of our dental practitioners surveyed would prescribe antibiotics routinely for periodontal abscess and pericoronitis. Most of those surveyed used antibiotics routinely for conditions where local treatment would be sufficient. Our findings indicate that the scientific basis for prescribing antimicrobial agents was neglected by the majority of the respondents. | Yes |
| Salako | The results of this study have demonstrated the lack of consistency in the rationale use of antibiotics. | Yes |
| Palmer | This survey supports the conclusion that there is overprescribing of antibiotics. | Yes |
| Author | Year | Study Type | Country | Number of Patients Treated for Pericoronitis (n) | Out of (n), Antibiotics Prescribed (An) | (An)/(n) in % |
|---|---|---|---|---|---|---|
| Combes * | 2019 | prospective | UK | 69 | 26 | 37.7 |
| Afghanistan | 478 | 183 | 38.3 | |||
| Bjelovucic | 2019 | retrospective | Croatia | 406 | 261 | 64.3 |
| Mahmoodi | 2015 | retrospective | Germany | 119 | 44 | 37.0 |
| Cope | 2016 | cross-sectional | UK | 72 | 67 | 93.1 |
| Tulip | 2008 | retrospective | UK | 46 | 39 | 84.8 |
| Total | 1190 | 620 | 52.1 | |||
| Note: | * | Dental care provision to UK military personnel serving in Afghanistan and at UK military home bases | ||||
| Author | Country | The Position of Pericoronitis in the Frequency of Antibiotic Use for Its Treatment within the Surveyed Diagnoses and Situations (n) | (n) |
|---|---|---|---|
| Combes | UK | 1st | ≥8 * |
| Afghanistan | 1st | ≥8 * | |
| Bjelovucic | Croatia | 2nd | 10 |
| Tulip | UK | 2nd | 14 |
| Cope | UK | 1st | 9 |
| Mahmoodi | Germany | 2nd | 5 |
| Note: | * | Total number of all diagnoses not clearly specified |
| Author | Country | Frequency of Prescribed Antibiotics | % |
|---|---|---|---|
| Combes | UK | not specified | |
| Afghanistan | |||
| Bjelovucic ** | Croatia | penicillin + clavulanic acid | 70.5 |
| clindamycin | 13.0 | ||
| metronidazole + penicillin | 7.2 | ||
| Tulip ** | UK | amoxicillin | 45.6 |
| metronidazole | 32.3 | ||
| Cope | UK | not specified | |
| Mahmoodi * | Germany | amoxicillin | 21.8 |
| amoxicillin + clavulanic acid | 10.9 | ||
| clindamycin | 3.4 | ||
| Notes: | * | Data for pericoronitis-related prescription | |
| ** | Data for all dental-related prescription | ||
| Author | Authors’ General Evaluation of Reported Treatments | Need for Further Education | |
|---|---|---|---|
| Combes | It could be argued that treatment of UK military personnel is predominantly more operative than their civilian counterparts… with reduced reliance on antibiotic therapy for the management of pericoronitis. | not stated | |
| Bjelovucic | Antibiotics were occasionally prescribed without dental treatment, namely in pericoronitis (46.3%). Multiple possible issues in the prescription of antibiotics were observed, ranging from administration for inappropriate indications to noncritical and excessive prescription. | yes | |
| Mahmoodi | Compared to the literature, surgical or dental interventions were more often performed with a more restrictive use of antibiotics. | not stated | |
| Cope | The current study demonstrated high levels of guideline-incongruent antibiotic prescribing by general dentist practitioners. Cases of pericoronitis, apical abscesses and acute periodontal conditions account for over 70% of all antibiotics prescribed (20.6% for pericoronitis). | yes | |
| Tulip | The study has highlighted that many GDPs are not familiar with current clinical and best practice guidelines on patient examination, management with respect to the correct prescribing of antibiotics for dental infections. | not stated * | |
| Note: | * | The authors stated that the reasons why dentists failed to provide definitive treatment and the high number of prescriptions for antibiotics require further research. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, J.; Kunderova, M.; Pilbauerova, N.; Kapitan, M. A Review of Evidence-Based Recommendations for Pericoronitis Management and a Systematic Review of Antibiotic Prescribing for Pericoronitis among Dentists: Inappropriate Pericoronitis Treatment Is a Critical Factor of Antibiotic Overuse in Dentistry. Int. J. Environ. Res. Public Health 2021, 18, 6796. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18136796
Schmidt J, Kunderova M, Pilbauerova N, Kapitan M. A Review of Evidence-Based Recommendations for Pericoronitis Management and a Systematic Review of Antibiotic Prescribing for Pericoronitis among Dentists: Inappropriate Pericoronitis Treatment Is a Critical Factor of Antibiotic Overuse in Dentistry. International Journal of Environmental Research and Public Health. 2021; 18(13):6796. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18136796
Chicago/Turabian StyleSchmidt, Jan, Martina Kunderova, Nela Pilbauerova, and Martin Kapitan. 2021. "A Review of Evidence-Based Recommendations for Pericoronitis Management and a Systematic Review of Antibiotic Prescribing for Pericoronitis among Dentists: Inappropriate Pericoronitis Treatment Is a Critical Factor of Antibiotic Overuse in Dentistry" International Journal of Environmental Research and Public Health 18, no. 13: 6796. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18136796