Follicle Loss and Apoptosis in Cyclophosphamide-Treated Mice: What’s the Matter?
Abstract
:1. Introduction
2. Results
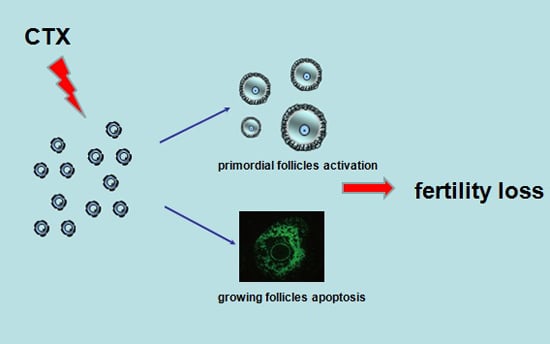
2.1. CyclophosphamideInduced Primordial Follicle Loss in Female Mice
2.2. Cyclophosphamide Accelerated Growing Follicle Apoptosis
2.3. The PI3K/Akt/mTOR Signaling Pathway May Participate in Ovarian Injury Due to CTX Treatment
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Ovarian Histomorphology
4.3. Immunohistochemical Analysis
4.4. Western Blot
4.5. TUNEL Assay
5. Conculsions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Loren, A.W.; Mangu, P.B.; Beck, L.N.; Brennan, L.; Magdalinski, A.J.; Partridge, A.H.; Quinn, G.; Wallace, W.H.; Oktay, K. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J. Clin. Oncol. 2013, 31, 2500–2510. [Google Scholar] [CrossRef] [PubMed]
- Meirow, D.; Dor, J.; Kaufman, B.; Shrim, A.; Rabinovici, J.; Schiff, E.; Raanani, H.; Levron, J.; Fridman, E. Gonadal dysfunction and fertility problems in cancer survivors. Acta. Oncol. 2007, 46, 480–489. [Google Scholar]
- Stroud, J.S.; Mutch, D.; Rader, J.; Powell, M.; Thaker, P.H.; Grigsby, P.W. Effects of cancer treatment on ovarian function. Fertil. Steril. 2009, 92, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Salama, M.; Winkler, K.; Murach, K.F.; Seeber, B.; Ziehr, S.C.; Wildt, L. Female fertility loss and preservation: Threats and opportunities. Ann. Oncol. 2013, 24, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Meirow, D.; Dor, J.; Kaufman, B.; Shrim, A.; Rabinovici, J.; Schiff, E.; Raanani, H.; Levron, J.; Fridman, E. Cortical fibrosis and blood-vessels damage in human ovaries exposed to chemotherapy. Potential mechanisms of ovarian injury. Hum. Reprod. 2007, 22, 1626–1633. [Google Scholar] [CrossRef] [PubMed]
- Meirow, D.; Biederman, H.; Anderson, R.A.; Wallace, W.H. Toxicity of chemotherapy and radiation on female reproduction. Clin. Obstet. Gynecol. 2010, 53, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Burnell, M.; Levine, M.N.; Chapman, J.A.; Bramwell, V.; Gelmon, K.; Walley, B.; Vandenberg, T.; Chalchal, H.; Albain, K.S.; Perez, E.A.; et al. Cyclophosphamide, epirubicin, and Fluorouracil versus dose-dense epirubicin and cyclophosphamide followed by Paclitaxel versus Doxorubicin and cyclophosphamide followed by Paclitaxel in node-positive or high-risk node-negative breast cancer. J. Clin. Oncol. 2010, 28, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Behringer, K.; Mueller, H.; Goergen, H.; Thielen, I.; Eibl, A.D.; Stumpf, V.; Wessels, C.; Wiehlpütz, M.; Rosenbrock, J.; Halbsguth, T.; et al. Gonadal function and fertility in survivors after Hodgkin lymphoma treatment within the German Hodgkin Study Group HD13 to HD15 trials. J. Clin. Oncol. 2013, 31, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Devi, H.P.; Mazumder, P.B. Methanolicextract of curcuma caesiaRoxb. Prevents the toxicity caused by cyclophosphamide to bone marrow cells, liver and kidney of mice. Pharmacogn. Res. 2016, 8, 43–49. [Google Scholar]
- Song, Y.; Zhang, C.; Wang, C.; Zhao, L.; Wang, Z.; Dai, Z.; Lin, S.; Kang, H.; Ma, X. Ferulicacid against cyclophosphamide-induced Heart toxicity in mice by inhibiting NF-κB pathway. Evid.-Based. Complement. Altern. Med. 2016, 2016, 1261270. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Turan, V.; Lierman, S.; Cuvelier, C.; de Sutter, P.; Oktay, K. Sphingosine-1-phosphate prevents chemotherapy-induced human primordial follicle death. Hum. Reprod. 2014, 29, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Blumenfeld, Z. Preservation of fertility and ovarian function and minimalization of chemotherapy associated gonadotoxicity and premature ovarian failure: The role of inhibin-A and -B as markers. Mol. Cell. Endocrinol. 2002, 187, 93–105. [Google Scholar] [CrossRef]
- Adhikari, D. In vitro activation of dormant follicles for fertility preservation. Adv. Exp. Med. Biol. 2013, 761, 29–42. [Google Scholar] [PubMed]
- Reddy, P.; Zheng, W.; Liu, K. Mechanisms maintaining the dormancy and survival of mammalian primordial follicles. Trends Endocrinol. Metab. 2010, 21, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.; Liu, L.; Adhikari, D.; Jagarlamudi, K.; Rajareddy, S.; Shen, Y.; Du, C.; Tang, W.; Hämäläinen, T.; Peng, S.L.; et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science 2008, 319, 611–613. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, D.; Risal, S.; Liu, K.; Shen, Y. Pharmacological inhibition of mTORC1 prevents over-activation of the primordial follicle pool in response to elevated PI3K signaling. PLoS ONE 2013, 8, e53810. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, D.; Zheng, W.; Shen, Y.; Gorre, N.; Hämäläinen, T.; Cooney, A.J.; Huhtaniemi, I.; Lan, Z.J.; Liu, K. Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles. Hum. Mol. Genet. 2010, 19, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Li, F.; Lu, Y.; Cao, Y.; Gao, J.; Liu, J. Rapamycin-sensitive mTORC1 signaling is involved in physiological primordial follicle activation in mouse ovary. Mol. Reprod. Dev. 2013, 80, 1018–1034. [Google Scholar] [CrossRef] [PubMed]
- El-Serafi, I.; Afsharian, P.; Moshfegh, A.; Hassan, M.; Terelius, Y. Cytochrome P450 oxidoreductase influences CYP2B6 activity in cyclophosphamide bioactivation. PLoS ONE 2015, 10, e0141979. [Google Scholar]
- Van Veelen, W.; Korsse, S.E.; van de Laar, L.; Peppelenbosch, M.P. The long and winding road to rational treatment of cancer associated with LKB1/AMPK/TSC/mTORC1 signaling. Oncogene 2011, 30, 2289–2303. [Google Scholar] [CrossRef] [PubMed]





© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.-Y.; Xia, H.-X.; Guan, H.-Y.; Li, B.; Zhang, W. Follicle Loss and Apoptosis in Cyclophosphamide-Treated Mice: What’s the Matter? Int. J. Mol. Sci. 2016, 17, 836. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17060836
Chen X-Y, Xia H-X, Guan H-Y, Li B, Zhang W. Follicle Loss and Apoptosis in Cyclophosphamide-Treated Mice: What’s the Matter? International Journal of Molecular Sciences. 2016; 17(6):836. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17060836
Chicago/Turabian StyleChen, Xiu-Ying, He-Xia Xia, Hai-Yun Guan, Bin Li, and Wei Zhang. 2016. "Follicle Loss and Apoptosis in Cyclophosphamide-Treated Mice: What’s the Matter?" International Journal of Molecular Sciences 17, no. 6: 836. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17060836