Characterisation and Antioxidant Activity of Crude Extract and Polyphenolic Rich Fractions from C. incanus Leaves
Abstract
:1. Introduction
2. Results and Discussion
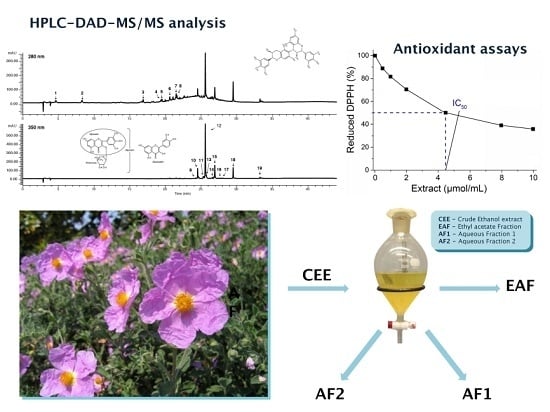
2.1. Qualitative Characterisation of Phenolic Compounds Present in Crude Extract of Cistus incanus (C. incanus) Leaves
2.2. Antiradical Activity Evaluation of Different Extracts of Cistus incanus (C. incanus) Leaves
2.2.1. Superoxide Anion Radical (O2 · −) and Hydroxyl Radical Scavenging Activities
2.2.2. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Radical Scavenging Activity
2.2.3. Structural Aspects of in Vitro Antiradical Activity of C. incanus Leaf Extracts
3. Materials and Methods
3.1. Plant Material and Extraction Procedure
3.2. Chemicals and Reagents
3.3. HPLC–DAD and LC–ESI-MS/MS Anlaysis of Phenolic Compounds
3.4. Superoxide Scavenging Activity
3.5. Hydroxyl Radical-Scavenging Activity
3.6. DPPH Radical-Scavenging Activity
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Martínez-Ferri, E.; Balaguer, L.; Valladares, F.; Chico, J.M.; Manrique, E. Energy dissipation in drought-avoiding and drought-tolerant tree species at midday during the Mediterranean summer. Tree Physiol. 2000, 20, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Di Ferdinando, M.; Brunetti, C.; Agati, G.; Tattini, M. Multiple functions of polyphenols in plants inhabiting unfavorable Mediterranean areas. Environ. Exp. Bot. 2014, 103, 107–116. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 3rd ed.; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Fink, R.C.; Scandalios, J.G. Molecular evolution and structure-function relationships of the superoxide dismutase gene families in Angiosperms and their relationship to other eukaryotic and prokaryotic superoxide dismutases. Arch. Biochem. Biophys. 2002, 399, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. How to characterize a biological antioxidant. Free Radic. Res. Commun. 1990, 9, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Grace, S.C.; Logan, B.A. Energy dissipation and radical scavenging by the plant phenylpropanoid pathway. Philos. Trans. R. Soc. Lond. 2000, 355, 1499–1510. [Google Scholar] [CrossRef] [PubMed]
- Triantaphylidès, C.; Havaux, M. Singlet oxygen in plants: Production, detoxification and signaling. Trends Plant Sci. 2009, 14, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Galleano, M.; Verstraeten, S.V.; Oteiza, P.I.; Fraga, C.G. Antioxidant actions of flavonoids: Thermodynamic and kinetic analysis. Arch. Biochem. Biophys. 2010, 501, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, C.; Di Ferdinando, M.; Fini, A.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants and developmental regulators: Relative significance in plants and humans. Int. J. Mol. Sci. 2013, 14, 3540–3555. [Google Scholar] [CrossRef] [PubMed]
- Pollastri, S.; Tattini, M. Flavonols: Old compounds for old roles. Ann. Bot. 2011, 108, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.X.; Kumamoto, T. Flavonoids as protein kinase inhibitors for cancer chemoprevention: Direct binding and molecular modeling. Antioxid. Redox Signal. 2010, 13, 691–719. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, S.; Gomes, D.; Costa, P.; Romano, A. The phenolic content and antioxidant activity of infusions from Mediterranean medicinal plants. Ind. Crops Prod. 2013, 43, 465–471. [Google Scholar] [CrossRef]
- Petereit, F.; Kolodziej, H.; Nahrstedt, A. Flavan-3-ols and proanthocyanidins from Cistus incanus. Phytochemistry 1991, 30, 981–985. [Google Scholar] [CrossRef]
- Danne, A.; Petereit, F.; Nahrstedt, A. Proanthocyanidins from Cistus incanus. Phytochemistry 1993, 34, 1129–1133. [Google Scholar] [CrossRef]
- Chinou, I.; Demetzos, C.; Harvala, C.; Roussakis, C.; Verbist, J. Cytotoxic and antibacterial labdane-type diterpenes from the aerial parts of Cistus incanus subsp. creticus. Planta Med. 1994, 60, 34–36. [Google Scholar] [CrossRef] [PubMed]
- Bouamama, H.; Noel, T.; Villard, J.; Benharref, A.; Jana, M. Antimicrobial activities of the leaf extracts of two Moroccan Cistus L. species. J. Ethnopharmacol. 2006, 104, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Wittpahl, G.; Kölling-Speer, I.; Basche, S.; Herrmann, E.; Hannig, M.; Speer, K.; Hannig, C. The polyphenolic composition of Cistus incanus herbal tea and its antibacterial and anti-adherent activity against Streptococcus mutans. Planta Med. 2015, 81, 1727–1735. [Google Scholar] [CrossRef] [PubMed]
- Droebner, K.; Ehrhardt, C.; Poetter, A.; Ludwig, S.; Planz, O. CYSTUS052, a polyphenol-rich plant extract, exerts anti-influenza virus activity in mice. Antivir. Res. 2007, 76, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rebensburg, S.; Helfer, M.; Schneider, M.; Koppensteiner, H.; Eberle, J.; Schindler, M.; Gürtler, L.; Brack-Werner, R. Potent in vitro antiviral activity of Cistus incanus extract against HIV and filoviruses targets viral envelope proteins. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Barrajón-Catalán, E.; Fernández-Arroyo, S.; Roldán, C.; Guillén, E.; Saura, D.; Segura-Carretero, A.; Micol, V. A systematic study of the polyphenolic composition of aqueous extracts deriving from several Cistus genus species: Evolutionary relationship. Phytochem. Anal. 2011, 22, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Riehle, P.; Vollmer, M.; Rohn, S. Phenolic compounds in Cistus incanus herbal infusions—Antioxidant capacity and thermal stability during the brewing process. Food Res. Int. 2013, 53, 891–899. [Google Scholar] [CrossRef]
- Santagati, N.A.; Salerno, L.; Attaguile, G.; Savoca, F.; Ronsisvalle, G. Simultaneous determination of catechins, rutin, and gallic acid in Cistus species extracts by HPLC with diode array detection. J. Chromatogr. Sci. 2008, 46, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, W.; Eberhardt, A.; Galensa, R. Investigation of proanthocyanidins by HPLC with electrospray ionization mass spectrometry. Eur. Food Res. Technol. 2000, 211, 56–64. [Google Scholar] [CrossRef]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Zhang, Z.; Beecher, G.; Holden, J.; Haytowitz, D.; Prior, R.L. Liquid chromatographic/electrospray ionization mass spectrometric studies of proanthocyanidins in foods. J. Mass Spectrom. 2003, 38, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Callemien, D.; Collin, S. Use of RP-HPLC-ESI(−)-MS/MS to differentiate various proanthocyanidin isomers in lager beer extracts. J. Am. Soc. Brew. Chem. 2008, 66, 109–115. [Google Scholar]
- Karonen, M.; Loponen, J.; Ossipov, V.; Pihlaja, K. Analysis of procyanidins in pine bark with reversed-phase and normal-phase high-performance liquid chromatography-electrospray ionization mass spectrometry. Anal. Chim. Acta 2004, 522, 105–112. [Google Scholar] [CrossRef]
- Gabetta, B.; Fuzzati, N.; Griffini, A.; Lolla, E.; Pace, R.; Ruffilli, T.; Peterlongo, F. Characterisation of proanthocyanidins from grape seeds. Fitoterapia 2000, 71, 162–175. [Google Scholar] [CrossRef]
- Saracini, E.; Tattini, M.; Traversi, M.; Vincieri, F.; Pinelli, P. Simultaneous LC-DAD and LC-MS determination of ellagitannins, flavonoid glycosides, and acyl-glycosyl flavonoids in Cistus salvifolius L. leaves. Chromatographia 2005, 62, 245–249. [Google Scholar] [CrossRef]
- Fracassetti, D.; Costa, C.; Moulay, L.; Tomás-Barberán, F.A. Ellagic acid derivatives, ellagitannins, proanthocyanidins and other phenolics, vitamin C and antioxidant capacity of two powder products from camu-camu fruit (Myrciaria dubia). Food Chem. 2013, 139, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pérez, C.; Quirantes-Piné, R.; Amessis-Ouchemoukh, N.; Madani, K.; Segura-Carretero, A.; Fernández-Gutierrez, A. A metabolite-profiling approach allows the identification of new compounds from Pistacia lentiscus leaves. J. Pharm. Biomed. Anal. 2013, 77, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J.; Bórquez, J.; Schmeda-Hirschmann, G. Antioxidant capacity, polyphenolic content and tandem HPLC–DAD-ESI/MS profiling of phenolic compounds from the South American berries Luma apiculata and L. Chequén. Food Chem. 2013, 139, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Ablajan, K.; Abliz, Z.; Shang, X.Y.; He, J.M.; Zhang, R.P.; Shi, J.G. Structural characterisation of flavonol 3,7-di-O-glycosides and determination of the glycosylation position by using negative ion electrospray ionization tandem mass spectrometry. J. Mass Spectrom. 2006, 41, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Dueñas, M.; Alves, C.T.; Silva, S.; Henriques, M.; Santos-Buelga, C.; Ferreira, I.C. Antifungal activity and detailed chemical characterisation of Cistus ladanifer phenolic extracts. Ind. Crops Prod. 2013, 41, 41–45. [Google Scholar] [CrossRef]
- Giusti, M.M.; Rodríguez-Saona, L.E.; Griffin, D.; Wrolstad, R.E. Electrospray and tandem mass spectroscopy as tools for anthocyanin characterisation. J. Agric. Food Chem. 1999, 47, 4657–4664. [Google Scholar] [CrossRef] [PubMed]
- Salaris, S.C.; Babbs, C.F.; Voorhees, W.D. Methylene blue as an inhibitor of superoxide generation by xanthine oxidase: A potential new drug for the attenuation of ischemia/reperfusion injury. Biochem. Pharmacol. 1991, 42, 499–506. [Google Scholar] [CrossRef]
- Cos, P.; Ying, L.; Calomme, M.; Hu, J.P.; Cimanga, K.; van Poel, B.; Berghe, D.V. Structure-activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J. Nat. Prod. 1998, 61, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Chun, O.K.; Kim, D.O.; Lee, C.Y. Superoxide radical scavenging activity of the major polyphenols in fresh plums. J. Agric. Food Chem. 2003, 51, 8067–8072. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, L.V.; Madsen, H.L.; Thomsen, M.K.; Dragsted, L.O.; Skibsted, L.H. Regeneration of phenolic antioxidants from phenoxyl radicals: An ESR and electrochemical study of antioxidant hierarchy. Free Radic. Res. 1999, 30, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Akkol, E.K.; Orhan, I.E.; Yeşilada, E. Anticholinesterase and antioxidant effects of the ethanol extract, ethanol fractions and isolated flavonoids from Cistus laurifolius L. leaves. Food Chem. 2012, 131, 626–631. [Google Scholar] [CrossRef]
- Tomás-Menor, L.; Morales-Soto, A.; Barrajón-Catalán, E.; Roldán-Segura, C.; Segura-Carretero, A.; Micol, V. Correlation between the antibacterial activity and the composition of extracts derived from various spanish Cistus species. Food Chem. Toxicol. 2013, 55, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Amić, D.; Davidović-Amić, D.; Bešlo, D.; Trinajstić, N. Structure-radical scavenging activity relationships of flavonoids. Croat. Chem. Acta 2003, 76, 55–61. [Google Scholar]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Faria, A.; Calhau, C.; de Freitas, V.; Mateus, N. Procyanidins as antioxidants and tumor cell growth modulators. J. Agric. Food Chem. 2006, 54, 2392–2397. [Google Scholar] [CrossRef] [PubMed]
- Saint-Cricq de Gaulejac, N.; Provost, C.; Vivas, N. Comparative study of polyphenol scavenging activities assessed by different methods. J. Agric. Food Chem. 1999, 47, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Hamada, Y.; Takano, S.; Ayano, Y.; Tokunaga, M.; Koashi, T.; Okamoto, S.; Doi, S.; Ishida, M.; Kawasaki, T.; Hamada, M. Structure–activity relationship of oligomeric flavan-3-ols: Importance of the upper-unit B-ring hydroxyl groups in the dimeric structure for strong activities. Molecules 2015, 20, 870–885. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Pinelli, P.; Mulinacci, N.; Vincieri, F.; Tattini, M. Identification and quantitation of polyphenols in leaves of Myrtus communis L. Chromatographia 1999, 49, 17–20. [Google Scholar] [CrossRef]
- Saucier, C.; Mirabel, M.; Daviaud, F.; Longieras, A.; Glories, Y. Rapid fractionation of grape seed proanthocyanidins. J. Agric. Food Chem. 2001, 49, 5732–5735. [Google Scholar] [CrossRef] [PubMed]
- Nishikimi, M.; Rao, N.A.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosul-phate and molecular oxygen. Biochem. Biophys. Res. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Smirnoff, N.; Cumbes, Q.J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 1989, 28, 1057–1060. [Google Scholar] [CrossRef]
- Baratto, M.C.; Tattini, M.; Galardi, C.; Pinelli, P.; Romani, A.; Visioli, F.; Basosi, R.; Pogni, R. Antioxidant activity of galloyl quinic derivatives isolated from P. Lentiscus leaves. Free Radic. Res. 2003, 37, 405–412. [Google Scholar] [CrossRef] [PubMed]





| Peak n | tR (min) | Λ (nm) | [M-H]− (m/z) | MS2 (m/z) | Tentative Assignement |
|---|---|---|---|---|---|
| 1 | 4.6 | 234,270 | 331 | 125, 169 | Monogalloyl glucose |
| 2 | 8.3 | 234,272 | 169 | 125 | Gallic acid |
| 3 | 16.9 | 236,272 | 609 | 441, 423, 483, 305, 303 | (Epi)Gallocatechin dimer |
| 4, 5 | 19.5 | 234,272 | 305 | 611, 125, 137 | (−)-Gallocatechin and (−)-epigallocatechin |
| 6 | 20.6 | 236,276 | 593 | 407, 467, 425, 289, 285 | (Epi)gallocatechin-(epi)catechin or (Epi)catechin-(epi)gallocatechin |
| 7, 8 | 21.5 | 236,278 | 289 | 245, 205 | (+) Catechin and (−) Epicatechin |
| 9 | 24.2 | 260,360 | n.d * | - | Myricetin derivative 1 |
| 10 | 24.5 | 254,362 | 479 | 316, 271 | Myricetin-3-O-hexoside |
| 11 | 25.4 | 260,360 | n.d * | - | Myricetin derivative 2 |
| 12 | 25.6 | 260,358 | 463 | 316, 271, 179 | Myricitrin |
| 13 | 25.7 | 256,356 | 609 | 301 | Rutin |
| 14 | 26.6 | 265,355 | 433 | 301, 271 | Quercetin-3-O-pentoside |
| 15 | 26.9 | 256,350 | 447 | 301, 179 | Quercitrin |
| 16 | 27.8 | 264,352 | n.d * | - | Quercetin derivative 1 |
| 17 | 28.2 | 264,352 | n.d * | - | Quercetin derivative 2 |
| 18 | 29.5 | 264,314,346sh | 593 | 285, 145 | Kaempferol 3-O-rutinoside |
| 19 | 33.3 | 268,314,348sh | 739 | 285, 306, 145, 452 | Kaempferol-3-(3″,6″-dicoumaroyl)-glucose |
| Sample | Monogalloyl Glucose and Gallic Acid | Catechins Derivatives a | Myricetin Derivatives b | Quercetin Derivatives c | Kaempferol Derivatives d | Proanthocyanidin Polymers |
|---|---|---|---|---|---|---|
| CEE | 0.315 ± 0.024 | 2.256 ± 0.076 | 2.719 ± 0.148 | 3.578 ± 0.217 | 0.055 ± 0.009 | 55.376 ± 3.067 |
| EAF | 0.236 ± 0.019 | 1.647 ± 0.069 | 2.202 ± 0.127 | 3.140 ± 0.162 | 0.036 ± 0.004 | nd |
| AF1 | nd | nd | nd | nd | nd | 25.193 ± 0.597 |
| AF2 | nd | nd | nd | nd | nd | 31.037 ± 0.901 |
| Sample | IC50 (μM) | ||
|---|---|---|---|
| Superoxide Anion Radical | Hydroxyl Radical | DPPH Radical | |
| CEE | 20.47 ± 1.05 b | 0.68 ± 0.05 c | 2.99 ± 1.18 b |
| EAF | 5.47 ± 0.98 d | 0.52 ± 0.05 d | 0.92 ± 0.10 c |
| AF1 | 24.99 ± 2.10 a | 0.99 ± 0.08 a | 11.78 ± 0.85 a |
| AF2 | 22.80 ± 1.19 a | 1.09 ± 0.12 a | 10.92 ± 0.38 a |
| MYR | 4.86 ± 0.86 d | 0.44 ± 0.03 d | 0.68 ± 0.07 c |
| EPI | 12.20 ± 1.65 c | 0.83 ± 0.07 b | 1.49 ± 0.27 b,c |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gori, A.; Ferrini, F.; Marzano, M.C.; Tattini, M.; Centritto, M.; Baratto, M.C.; Pogni, R.; Brunetti, C. Characterisation and Antioxidant Activity of Crude Extract and Polyphenolic Rich Fractions from C. incanus Leaves. Int. J. Mol. Sci. 2016, 17, 1344. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17081344
Gori A, Ferrini F, Marzano MC, Tattini M, Centritto M, Baratto MC, Pogni R, Brunetti C. Characterisation and Antioxidant Activity of Crude Extract and Polyphenolic Rich Fractions from C. incanus Leaves. International Journal of Molecular Sciences. 2016; 17(8):1344. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17081344
Chicago/Turabian StyleGori, Antonella, Francesco Ferrini, Maria Cristina Marzano, Massimiliano Tattini, Mauro Centritto, Maria Camilla Baratto, Rebecca Pogni, and Cecilia Brunetti. 2016. "Characterisation and Antioxidant Activity of Crude Extract and Polyphenolic Rich Fractions from C. incanus Leaves" International Journal of Molecular Sciences 17, no. 8: 1344. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17081344