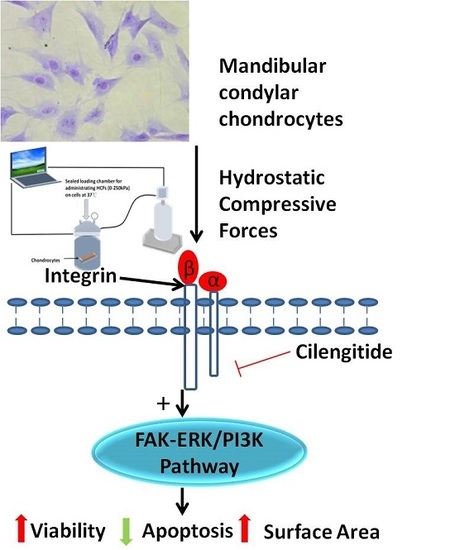
Hydrostatic Compress Force Enhances the Viability and Decreases the Apoptosis of Condylar Chondrocytes through Integrin-FAK-ERK/PI3K Pathway
Abstract
:1. Introduction
2. Results
2.1. Characterization of Primary Condylar Chondrocytes
2.2. HCFs Could Promote the Proliferation and Decrease the Apoptosis of Condylar Chondrocytes
2.3. HCFs Enlarged the Surface Area of Condylar Chondrocytes without Changing Their Chondrocyte Phenotype
2.4. Change of Integrin α2, Integrin α5, Integrin β1, FAK, ERK1/2, and PI3K under Various HCFs
3. Discussion
4. Materials and Methods
4.1. Isolation and Culture of Primary Mandibular Condylar Chondrocytes
4.2. Application of Hydrostatic Compressive Forces and Integrin Inhibitor
4.3. Cell Viability Assay
4.4. Detection of Cell Apoptosis
4.5. Actin Staining and Estimation of Cell Surface Area
4.6. Quantitative RT-PCR
4.7. Western Blot Analysis
4.8. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chai, Y.; Maxson, R.E., Jr. Recent advances in craniofacial morphogenesis. Dev. Dyn. 2006, 235, 2353–2375. [Google Scholar] [CrossRef]
- Hinton, R.J. Effect of condylotomy on matrix synthesis and mineralization in the rat mandibular condylar cartilage. Arch. Oral Biol. 1989, 34, 1003–1009. [Google Scholar] [CrossRef]
- Kantomaa, T.; Pirttiniemi, P.; Tuominen, M.; Poikela, A. Glycosaminoglycan synthesis in the mandibular condyle during growth adaptation. Acta Anat. 1994, 151, 88–96. [Google Scholar] [CrossRef]
- Kiliaridis, S.; Thilander, B.; Kjellberg, H.; Topouzelis, N.; Zafiriadis, A. Effect of low masticatory function on condylar growth: A morphometric study in the rat. Am. J. Orthod. Dentofac. Orthop. 1999, 116, 121–125. [Google Scholar] [CrossRef]
- Bouvier, M. Variation in alkaline-phosphatase activity with changing load on the mandibular condylar cartilage in the rat. Arch. Oral Biol. 1987, 32, 671–675. [Google Scholar] [CrossRef]
- Hinton, R.J.; Carlson, D.S. Response of the mandibular joint to loss of incisal function in the rat. Acta Anat. 1986, 125, 145–151. [Google Scholar] [CrossRef]
- Pirttiniemi, P.; Kantomaa, T.; Salo, L.; Tuominen, M. Effect of reduced articular function on deposition of type I and type II collagens in the mandibular condylar cartilage of the rat. Arch. Oral Biol. 1996, 41, 127–131. [Google Scholar] [CrossRef]
- Habib, H.; Hatta, T.; Udagawa, J.; Zhang, L.; Yoshimura, Y.; Otani, H. Fetal jaw movement affects condylar cartilage development. J. Dent. Res. 2005, 84, 474–479. [Google Scholar] [CrossRef]
- Pirttiniemi, P.; Kantomaa, T.; Sorsa, T. Effect of decreased loading on the metabolic activity of the mandibular condylar cartilage in the rat. Eur. J. Orthod. 2004, 26, 1–5. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, S.T.; Cho, S.W.; Jung, H.S.; Park, K.T.; Son, H.K. Growth effects of botulinum toxin type A injected into masseter muscle on a developing rat mandible. Oral Dis. 2008, 14, 626–632. [Google Scholar] [CrossRef]
- Jung, J.K.; Sohn, W.J.; Lee, Y.; Bae, Y.C.; Choi, J.K.; Kim, J.Y. Morphological and cellular examinations of experimentally induced malocclusion in mice mandibular condyle. Cell Tissue Res. 2014, 355, 355–363. [Google Scholar] [CrossRef]
- Humphries, M.J. Integrin structure. Biochem. Soc. Trans. 2000, 28, 311–339. [Google Scholar] [CrossRef]
- Woods, V.L., Jr.; Schreck, P.J.; Gesink, D.S.; Pacheco, H.O.; Amiel, D.; Akeson, W.H.; Lotz, M. Integrin expression by human articular chondrocytes. Arthritis Rheum. 1994, 37, 537–544. [Google Scholar] [CrossRef]
- Wright, M.O.; Nishida, K.; Bavington, C.; Godolphin, J.L.; Dunne, E.; Walmsley, S.; Jobanputra, P.; Nuki, G.; Salter, D.M. Hyperpolarisation of cultured human chondrocytes following cyclical pressure-induced strain: Evidence of a role for α5β1 integrin as a chondrocyte mechanoreceptor. J. Orthop. Res. 1997, 15, 742–747. [Google Scholar] [CrossRef]
- Luo, D.Y.; Wazir, R.; Tian, Y.; Yue, X.; Wei, T.Q.; Wang, K.J. Integrin αv mediates contractility whereas integrin α4 regulates proliferation of human bladder smooth muscle cells via FAK pathway under physiological stretch. J. Urol. 2013, 190, 1421–1429. [Google Scholar] [CrossRef]
- Ju, L.; Zhou, C. Association of integrin β1 and c-MET in mediating EGFR TKI gefitinib resistance in non-small cell lung cancer. Cancer Cell Int. 2013, 13, 15. [Google Scholar] [CrossRef]
- Luu, N.T.; Glen, K.E.; Egginton, S.; Rainger, G.E.; Nash, G.B. Integrin-substrate interactions underlying shear-induced inhibition of the inflammatory response of endothelial cells. Thromb. Haemost. 2013, 109, 298–308. [Google Scholar] [CrossRef]
- Liu, C.; Kaneko, S.; Soma, K. Expression of integrin α5β1, focal adhesion kinase and integrin-linked kinase in rat condylar cartilage during mandibular lateral displacement. Arch. Oral Biol. 2008, 53, 701–708. [Google Scholar] [CrossRef]
- Hirsch, M.S.; Lunsford, L.E.; Trinkaus-Randall, V.; Svoboda, K.K. Chondrocyte survival and differentiation in situ are integrin mediated. Dev. Dyn. 1997, 210, 249–263. [Google Scholar] [CrossRef]
- Hehlgans, S.; Haase, M.; Cordes, N. Signalling via integrins: Implications for cell survival and anticancer strategies. Biochim. Biophys. Acta 2007, 1775, 163–180. [Google Scholar] [CrossRef]
- Diercke, K.; Kohl, A.; Lux, C.J.; Erber, R. Strain-dependent up-regulation of ephrin-B2 protein in periodontal ligament fibroblasts contributes to osteogenesis during tooth movement. J. Biol. Chem. 2011, 286, 37651–37664. [Google Scholar] [CrossRef]
- Hong, S.Y.; Jeon, Y.M.; Lee, H.J.; Kim, J.G.; Baek, J.A.; Lee, J.C. Activation of rhoa and FAK induces ERK-mediated osteopontin expression in mechanical force-subjected periodontal ligament fibroblasts. Mol. Cell. Biochem. 2010, 335, 263–272. [Google Scholar] [CrossRef]
- Ory, S.; Morrison, D.K. Signal transduction: Implications for Ras-dependent ERK signaling. Curr. Biol. 2004, 14, R277–R278. [Google Scholar] [CrossRef]
- Sonoda, Y.; Matsumoto, Y.; Funakoshi, M.; Yamamoto, D.; Hanks, S.K.; Kasahara, T. Anti-apoptotic role of focal adhesion kinase (FAK). Induction of inhibitor-of-apoptosis proteins and apoptosis suppression by the overexpression of FAK in a human leukemic cell line, HL-60. J. Biol. Chem. 2000, 275, 16309–16315. [Google Scholar] [CrossRef]
- Nishio, C.; Tanimoto, K.; Hirose, M.; Horiuchi, S.; Kuroda, S.; Tanne, K.; Tanaka, E. Stress analysis in the mandibular condyle during prolonged clenching: A theoretical approach with the finite element method. Proc. Inst. Mech. Eng. H 2009, 223, 739–748. [Google Scholar] [CrossRef]
- Abe, S.; Kawano, F.; Kohge, K.; Kawaoka, T.; Ueda, K.; Hattori-Hara, E.; Mori, H.; Kuroda, S.; Tanaka, E. Stress analysis in human temporomandibular joint affected by anterior disc displacement during prolonged clenching. J. Oral Rehabil. 2013, 40, 239–246. [Google Scholar] [CrossRef]
- Liang, W.; Ren, K.; Liu, F.; Cui, W.; Wang, Q.; Chen, Z.; Fan, W. Periodic mechanical stress stimulates the FAK mitogenic signal in rat chondrocytes through ERK1/2 activity. Cell. Physiol. Biochem. 2013, 32, 915–930. [Google Scholar] [CrossRef]
- Lucchinetti, E.; Adams, C.S.; Horton, W.E., Jr.; Torzilli, P.A. Cartilage viability after repetitive loading: A preliminary report. Osteoarthr. Cartil. 2002, 10, 71–81. [Google Scholar] [CrossRef]
- Chen, C.T.; Bhargava, M.; Lin, P.M.; Torzilli, P.A. Time, stress, and location dependent chondrocyte death and collagen damage in cyclically loaded articular cartilage. J. Orthop. Res. 2003, 21, 888–898. [Google Scholar] [CrossRef]
- Tatsumura, M.; Sakane, M.; Ochiai, N.; Mizuno, S. Off-loading of cyclic hydrostatic pressure promotes production of extracellular matrix by chondrocytes. Cells Tissues Org. 2013, 198, 405–413. [Google Scholar] [CrossRef]
- Fioravanti, A.; Cantarini, L.; Chellini, F.; Manca, D.; Paccagnini, E.; Marcolongo, R.; Collodel, G. Effect of hyaluronic acid (MW 500–730 kDa) on proteoglycan and nitric oxide production in human osteoarthritic chondrocyte cultures exposed to hydrostatic pressure. Osteoarthr. Cartil. 2005, 13, 688–696. [Google Scholar] [CrossRef]
- Pascarelli, N.A.; Collodel, G.; Moretti, E.; Cheleschi, S.; Fioravanti, A. Changes in ultrastructure and cytoskeletal aspects of human normal and osteoarthritic chondrocytes exposed to interleukin-1β and cyclical hydrostatic pressure. Int. J. Mol. Sci. 2015, 16, 26019–26034. [Google Scholar] [CrossRef]
- Huang, L.; Li, M.; Li, H.; Yang, C.; Cai, X. Study of differential properties of fibrochondrocytes and hyaline chondrocytes in growing rabbits. Br. J. Oral Maxillofac. Surg. 2015, 53, 187–193. [Google Scholar] [CrossRef]
- Takano-Yamamoto, T.; Soma, S.; Nakagawa, K.; Kobayashi, Y.; Kawakami, M.; Sakuda, M. Comparison of the effects of hydrostatic compressive force on glycosaminoglycan synthesis and proliferation in rabbit chondrocytes from mandibular condylar cartilage, nasal septum, and spheno-occipital synchondrosis in vitro. Am. J. Orthod. Dentofac. Orthop. 1991, 99, 448–455. [Google Scholar] [CrossRef]
- Cao, L.; Lee, V.; Adams, M.E.; Kiani, C.; Zhang, Y.; Hu, W.; Yang, B.B. β1-Integrin-collagen interaction reduces chondrocyte apoptosis. Matrix Biol. 1999, 18, 343–355. [Google Scholar] [CrossRef]
- Ren, K.; Liu, F.; Huang, Y.; Liang, W.; Cui, W.; Wang, Q.; Fan, W. Periodic mechanical stress activates integrinβ1-dependent Src-dependent PLCγ1-independent Rac1 mitogenic signal in rat chondrocytes through ERK1/2. Cell. Physiol. Biochem. 2012, 30, 827–842. [Google Scholar] [CrossRef]
- Ross, R.S. The extracellular connections: The role of integrins in myocardial remodeling. J. Card. Fail. 2002, 8, S326–S331. [Google Scholar] [CrossRef]
- McGill, G.; Shimamura, A.; Bates, R.C.; Savage, R.E.; Fisher, D.E. Loss of matrix adhesion triggers rapid transformation-selective apoptosis in fibroblasts. J. Cell Biol. 1997, 138, 901–911. [Google Scholar] [CrossRef]
- Wen, H.; Blume, P.A.; Sumpio, B.E. Role of integrins and focal adhesion kinase in the orientation of dermal fibroblasts exposed to cyclic strain. Int. Wound J. 2009, 6, 149–158. [Google Scholar] [CrossRef]
- Lal, H.; Verma, S.K.; Smith, M.; Guleria, R.S.; Lu, G.; Foster, D.M.; Dostal, D.E. Stretch-induced MAP kinase activation in cardiac myocytes: Differential regulation through β1-integrin and focal adhesion kinase. J. Mol. Cell. Cardiol. 2007, 43, 137–147. [Google Scholar] [CrossRef]
- Frisch, S.M.; Vuori, K.; Ruoslahti, E.; Chan-Hui, P.Y. Control of adhesion-dependent cell survival by focal adhesion kinase. J. Cell Biol. 1996, 134, 793–799. [Google Scholar] [CrossRef]
- Hungerford, J.E.; Compton, M.T.; Matter, M.L.; Hoffstrom, B.G.; Otey, C.A. Inhibition of pp125FAK in cultured fibroblasts results in apoptosis. J. Cell Biol. 1996, 135, 1383–1390. [Google Scholar] [CrossRef]
- Liao, C.H.; Sang, S.; Ho, C.T.; Lin, J.K. Garcinol modulates tyrosine phosphorylation of FAK and subsequently induces apoptosis through down-regulation of Src, ERK, and AKT survival signaling in human colon cancer cells. J. Cell. Biochem. 2005, 96, 155–169. [Google Scholar] [CrossRef]
- Schlaepfer, D.D.; Hanks, S.K.; Hunter, T.; van der Geer, P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature 1994, 372, 786–791. [Google Scholar] [CrossRef]
- Mobasheri, A.; Carter, S.D.; Martin-Vasallo, P.; Shakibaei, M. Integrins and stretch activated ion channels; putative components of functional cell surface mechanoreceptors in articular chondrocytes. Cell Biol. Int. 2002, 26, 1–18. [Google Scholar] [CrossRef]
- Shakibaei, M.; Schulze-Tanzil, G.; de Souza, P.; John, T.; Rahmanzadeh, M.; Rahmanzadeh, R.; Merker, H.J. Inhibition of mitogen-activated protein kinase kinase induces apoptosis of human chondrocytes. J. Biol. Chem. 2001, 276, 13289–13294. [Google Scholar] [CrossRef]
- Kiyokawa, E.; Hashimoto, Y.; Kurata, T.; Sugimura, H.; Matsuda, M. Evidence that DOCK180 up-regulates signals from the CrkII-p130(Cas) complex. J. Biol. Chem. 1998, 273, 24479–24484. [Google Scholar] [CrossRef]
- Chen, H.C.; Guan, J.L. Association of focal adhesion kinase with its potential substrate phosphatidylinositol 3-kinase. Proc. Natl. Acad. Sci. USA 1994, 91, 10148–10152. [Google Scholar] [CrossRef]
- Buckley, C.D.; Pilling, D.; Henriquez, N.V.; Parsonage, G.; Threlfall, K.; Scheel-Toellner, D.; Simmons, D.L.; Akbar, A.N.; Lord, J.M.; Salmon, M. RGD peptides induce apoptosis by direct caspase-3 activation. Nature 1999, 397, 534–539. [Google Scholar]
- Folkman, J.; Moscona, A. Role of cell shape in growth control. Nature 1978, 273, 345–349. [Google Scholar] [CrossRef]
- Gospodarowicz, D.; Greenburg, G.; Birdwell, C.R. Determination of cellular shape by the extracellular matrix and its correlation with the control of cellular growth. Cancer Res. 1978, 38, 4155–4171. [Google Scholar]
- Engel, F.E.; Khare, A.G.; Boyan, B.D. Phenotypic changes of rabbit mandibular condylar cartilage cells in culture. J. Dent. Res. 1990, 69, 1753–1758. [Google Scholar] [CrossRef]
- Adams, S.L.; Pallante, K.M.; Pacifici, M. Effects of cell shape on type x collagen gene expression in hypertrophic chondrocytes. Connect. Tissue Res. 1989, 20, 223–232. [Google Scholar] [CrossRef]
- Gerstenfeld, L.C.; Kelly, C.M.; von Deck, M.; Lian, J.B. Comparative morphological and biochemical analysis of hypertrophic, non-hypertrophic and L, 25(OH)2d3 treated non-hypertrophic chondrocytes. Connect. Tissue Res. 1990, 24, 29–39. [Google Scholar] [CrossRef]
- Bonassar, L.J.; Grodzinsky, A.J.; Srinivasan, A.; Davila, S.G.; Trippel, S.B. Mechanical and physicochemical regulation of the action of insulin-like growth factor-I on articular cartilage. Arch. Biochem. Biophys. 2000, 379, 57–63. [Google Scholar] [CrossRef]
- Grodzinsky, A.J.; Levenston, M.E.; Jin, M.; Frank, E.H. Cartilage tissue remodeling in response to mechanical forces. Annu. Rev. Biomed. Eng. 2000, 2, 691–713. [Google Scholar] [CrossRef]
- Sanz-Ramos, P.; Mora, G.; Ripalda, P.; Vicente-Pascual, M.; Izal-Azcarate, I. Identification of signalling pathways triggered by changes in the mechanical environment in rat chondrocytes. Osteoarthr. Cartil. 2012, 20, 931–939. [Google Scholar] [CrossRef]
- Seguin, C.A.; Bernier, S.M. TNFα suppresses link protein and type II collagen expression in chondrocytes: Role of MEK1/2 and NF-κB signaling pathways. J. Cell. Physiol. 2003, 197, 356–369. [Google Scholar] [CrossRef]
- Takigawa, M.; Takano, T.; Suzuki, F. Restoration by cyclic AMP of the differentiated phenotype of chondrocytes from de-differentiated cells pretreated with retinoids. Mol. Cell. Biochem. 1982, 42, 145–153. [Google Scholar] [CrossRef]
- Wu, M.J.; Gu, Z.Y.; Sun, W. Effects of hydrostatic pressure on cytoskeleton and BMP-2, TGF-Β, SOX-9 production in rat temporomandibular synovial fibroblasts. Osteoarthr. Cartil. 2008, 16, 41–47. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kita, M.; Kimura, I.; Oseko, F.; Terauchi, R.; Takahashi, K.; Kubo, T.; Kanamura, N. Mechanical stress induces expression of cytokines in human periodontal ligament cells. Oral Dis. 2006, 12, 171–175. [Google Scholar] [CrossRef]
- Smith, R.L.; Rusk, S.F.; Ellison, B.E.; Wessells, P.; Tsuchiya, K.; Carter, D.R.; Caler, W.E.; Sandell, L.J.; Schurman, D.J. In vitro stimulation of articular chondrocyte mrna and extracellular matrix synthesis by hydrostatic pressure. J. Orthop. Res. 1996, 14, 53–60. [Google Scholar] [CrossRef]
- Ozawa, H.; Imamura, K.; Abe, E.; Takahashi, N.; Hiraide, T.; Shibasaki, Y.; Fukuhara, T.; Suda, T. Effect of a continuously applied compressive pressure on mouse osteoblast-like cells (MC3T3-E1) in vitro. J. Cell. Physiol. 1990, 142, 177–185. [Google Scholar] [CrossRef]
- Oliveira-Ferrer, L.; Hauschild, J.; Fiedler, W.; Bokemeyer, C.; Nippgen, J.; Celik, I.; Schuch, G. Cilengitide induces cellular detachment and apoptosis in endothelial and glioma cells mediated by inhibition of FAK/Src/AKT pathway. J. Exp. Clin. Cancer Res. 2008, 27, 86. [Google Scholar] [CrossRef]
- Bae, J.Y.; Han, D.W.; Wakitani, S.; Nawata, M.; Hyon, S.H. Biological and biomechanical evaluations of osteochondral allografts preserved in cold storage solution containing epigallocatechin gallate. Cell Transplant. 2010, 19, 681–689. [Google Scholar] [CrossRef]
- Mayr, M.; Li, C.; Zou, Y.; Huemer, U.; Hu, Y.; Xu, Q. Biomechanical stress-induced apoptosis in vein grafts involves p38 mitogen-activated protein kinases. FASEB J. 2000, 14, 261–270. [Google Scholar]
- Cruz-Orive, L.M.; Weibel, E.R. Recent stereological methods for cell biology: A brief survey. Am. J. Physiol. 1990, 258, L148–L156. [Google Scholar]









| Gene | Accession No. | Primers (5′-3′) (F = Forward; R = Reverse) |
|---|---|---|
| Itga2 | XM_001075558.5 | F: TGGAATTTGTTCTGATGTCAGTCC |
| R: GTGGTCAAGTTAAAGACAACTCTT | ||
| Itga5 | NM_001314041.1 | F: TGCAGCACCATTCAATTTGACAGC |
| R: TCATTCTGTGGGTCCTTTTCTGTG | ||
| Itgb1 | XM_006255824.1 | F: AATTCAAGAGGGCTGAAGACTAC |
| R: TGTCAGTAAGACTAAGCACG | ||
| FAK | XM_006520433.2 | F: CCAAGTTCGAGTACTAAGACTCACC |
| R: AAATCCATAGCAGGCCACGTGC | ||
| ERK1 | NM_017347.2 | F: AGAGATCATGCTTAACTCCAAG |
| R: TTCATGTTAATGATACAATTTAGGTCCTC | ||
| ERK2 | XM_006522147.3 | F: CTGCACCGTGACCTCAAGCC |
| R: CAATGGACTTGGTATAACCCTTGG | ||
| PI3K1 | XM_006240005.2 | F: CTGATTGGCTACGACGTCAC |
| R: GAAGAAGCTCTGAAGGATGGTGTC | ||
| GAPDH | XM_017321385.1 | F: AAAGGCATCTTGGGCTACACCG |
| R: ATGAGGTCCACCACCCTGTTG |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, D.; Kou, X.; Jin, J.; Xu, T.; Wu, M.; Deng, L.; Fu, L.; Liu, Y.; Wu, G.; Lu, H. Hydrostatic Compress Force Enhances the Viability and Decreases the Apoptosis of Condylar Chondrocytes through Integrin-FAK-ERK/PI3K Pathway. Int. J. Mol. Sci. 2016, 17, 1847. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17111847
Ma D, Kou X, Jin J, Xu T, Wu M, Deng L, Fu L, Liu Y, Wu G, Lu H. Hydrostatic Compress Force Enhances the Viability and Decreases the Apoptosis of Condylar Chondrocytes through Integrin-FAK-ERK/PI3K Pathway. International Journal of Molecular Sciences. 2016; 17(11):1847. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17111847
Chicago/Turabian StyleMa, Dandan, Xiaoxing Kou, Jing Jin, Taotao Xu, Mengjie Wu, Liquan Deng, Lusi Fu, Yi Liu, Gang Wu, and Haiping Lu. 2016. "Hydrostatic Compress Force Enhances the Viability and Decreases the Apoptosis of Condylar Chondrocytes through Integrin-FAK-ERK/PI3K Pathway" International Journal of Molecular Sciences 17, no. 11: 1847. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17111847