Applications of Alginate-Based Bioinks in 3D Bioprinting
Abstract
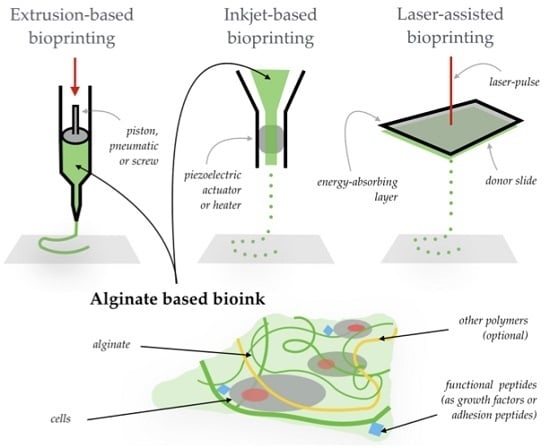
:1. Introduction
2. The Use of Alginate in Three-Dimensional (3D) Bioprinting
2.1. 3D Bioprinted Vascular Tissues
2.2. Bone Printing
2.3. Cartilage Printing
2.4. Other Advances in 3D Bioprinting
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Devillard, R.; Pagès, E.; Correa, M.M.; Kériquel, V.; Rémy, M.; Kalisky, J.; Ali, M.; Guillotine, B.; Guillemot, F. Cell patterning by laser-assisted bioprinting. Methods Cell Biol. 2014, 119, 159–174. [Google Scholar] [PubMed]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Sari, D.P.; Bang, S.; Nguyen, L.; Cho, Y.; Park, K.D.; Lee, S.; Lee, I.; Zhang, S.; Noh, I. Micro/nano surface topography and 3D bioprinting of biomaterials in tissue engineering. J. Nanosci. Nanotechnol. 2016, 16, 8909–8922. [Google Scholar] [CrossRef]
- Tay, C.Y.; Muthu, M.S.; Chia, S.L.; Nguyen, K.T.; Feng, S.S.; Leong, D.T. Reality check for nanomaterial-mediated therapy with 3D biomimetic culture systems. Adv. Funct. Mater. 2016, 26, 4046–4065. [Google Scholar] [CrossRef]
- Ma, X.; Qu, X.; Zhu, W.; Li, Y.S.; Yuan, S.; Zhang, H.; Liu, Y.; Wang, P.; Lai, C.S.E.; Zanella, F.; et al. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc. Natl. Acad. Sci. USA 2016, 113, 2206–2211. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.W.; Lee, S.J.; Ko, I.K.; Kengla, C.; Yoo, J.J.; Atala, A. A 3D biorprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016, 34, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.S. The billion cell construct: Will three-dimensional printing get us there? PLoS Biol. 2014, 12, e1001882. [Google Scholar] [CrossRef] [PubMed]
- Muraoka, M.; Shimizu, T.; Itoga, K.; Takahashi, H.; Okano, T. Control of the formation of vascular networks in 3D tissue engineered constructs. Biomaterials 2013, 34, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Hölzl, K.; Lin, S.; Tytgat, L.; van Vlierberghe, S.; Gu, L.; Ovsianikov, A. Bioink properties before, during and after 3D bioprinting. Biofabrication 2016, 8, 032002. [Google Scholar] [CrossRef] [PubMed]
- Stanton, M.M.; Samitier, J.; Sánchez, S. Bioprinting of 3D hydrogels. Lab Chip 2015, 15, 3111–3115. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Tønnesen, H.H.; Karlsen, J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Kulseng, B.; Skjåk-Braek, G.; Ryan, L.; Andersson, A.; King, A.; Faxvaag, A.; Espevik, T. Transplantation of alginate microcapsules: Generation of antibodies against alginates and encapsulated porcine islet-like cell clusters. Transplantation 1999, 67, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Leone, G.; Torricelli, P.; Chiumiento, A.; Facchini, A.; Barbucci, R. Amidic alginate hydrogel for nucleus pulposus replacement. J. Biomed. Mater. Res. A 2008, 84, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Fedorovich, N.E.; Schuurman, W.; Wijnberg, H.M.; Prins, H.J.; van Weeren, P.R.; Malda, J.; Alblas, J.; Dhert, W.J. Biofabrication of osteochondral tissue equivalents by printing topologically defined, cell-laden hydrogel scaffolds. Tissue Eng. C Methods 2012, 18, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Lim, F.; Sun, A.M. Microencapsulated islets as bioartificial endocrine pancreas. Science 1980, 210, 908–910. [Google Scholar] [CrossRef] [PubMed]
- O’shea, G.M.; Sun, A.M. Encapsulation of rat islets of Langerhans prolongs xenograft survival in diabetic mice. Diabetes 1986, 35, 943–946. [Google Scholar] [CrossRef] [PubMed]
- Gombotz, W.R.; Wee, S.F. Protein release from alginate matrices. Adv. Drug Deliv. Rev. 1998, 31, 267–285. [Google Scholar] [CrossRef]
- Grigore, A.; Sarker, B.; Fabry, B.; Boccaccini, A.R.; Detsch, R. Behavior of Encapsulated MG-63 Cells in RGD and Gelatine-Modified Alginate Hydrogels. Tissue Eng. A 2014, 20, 2140–2150. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Richards, D.J.; Pollard, S.; Tan, Y.; Rodriguez, J.; Visconti, R.P.; Trusk, T.C.; Yost, M.J.; Yao, H.; Markwald, R.R.; et al. Engineering alginate as bioink for bioprinting. Acta Biomater. 2014, 10, 4323–4331. [Google Scholar] [CrossRef] [PubMed]
- Boontheekul, T.; Kong, H.-J.; Mooney, D.J. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials 2005, 26, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.J.; Kaigler, D.; Kim, K.; Mooney, D.J. Controlling rigidity and degradation of alginate hydrogels via molecular weight distribution. Biomacromolecules 2004, 5, 1720–1727. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Preston, L.A.; Schiller, N.L. ALGINATE LYASE: Review of major sources and enzyme characteristics, structure-function analysis, biological roles, and applications. Annu. Rev. Microbiol. 2000, 54, 289–340. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J.; Hochberg, M. Self-regulation of growth in three dimensions. J. Exp. Med. 1973, 138, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Auger, F.A.; Gibot, L.; Lacroix, D. The pivotal role of vascularization in tissue engineering. Annu. Rev. Biomed. Eng. 2013, 15, 177–200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, Y.; Chen, H.; Ozbolat, I.T. Characterization of printable cellular micro-fluidic channels for tissue engineering. Biofabrication 2013, 5, 025004. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, Y.; Martin, J.A.; Ozbolat, I.T. Evaluation of cell viability and functionality in vessel-like bioprintable cell-laden tubular channels. J. Biomech. Eng. 2013, 135. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; He, Y.; Fu, J.-Z.; Liu, A.; Ma, L. Coaxial nozzle-assisted 3D bioprinting with built-in microchannels for nutrients delivery. Biomaterials 2015, 61, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Gungor-Ozkerim, P.S.; Zhang, Y.S.; Yue, K.; Zhu, K.; Liu, W.; Pi, Q.; Byambaa, B.; Dokmeci, M.R.; Shin, S.R.; et al. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials 2016, 106, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.; Xu, C.; Chai, W.; Zhang, Z.; Fu, J.; Huang, Y. Freeform inkjet printing of cellular structures with bifurcations. Biotechnol. Bioeng. 2015, 112, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Wüst, S.; Godla, M.E.; Müller, R.; Hofmann, S. Tunable hydrogel composite with two-step processing in combination with innovative hardware upgrade for cell-based three-dimensional bioprinting. Acta Biomater. 2014, 10, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.-H.; Lee, J.-S.; Kim, J.Y.; Cho, D.-W. Bioprinting of a mechanically enhanced three-dimensional dual cell-laden construct for osteochondral tissue engineering using a multi-head tissue/organ building system. J. Micromech. Microeng. 2012, 22. [Google Scholar] [CrossRef]
- Armstrong, J.P.K.; Burke, M.; Carter, B.M.; Davis, S.A.; Perriman, A.W. 3D Bioprinting Using a Templated Porous Bioink. Adv. Healthc. Mater. 2016, 5, 1724–1730. [Google Scholar] [CrossRef] [PubMed]
- Neufurth, M.; Wang, X.; Schröder, H.C.; Feng, Q.; Diehl-Seifert, B.; Ziebart, T.; Steffen, R.; Wang, S.; Müller, W.E.G. Engineering a morphogenetically active hydrogel for bioprinting of bioartificial tissue derived from human osteoblast-like SaOS-2 cells. Biomaterials 2014, 35, 8810–8819. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tolba, E.; Der, H.C.S.; Neufurth, M.; Feng, Q.; Diehl-Seifert, B.R.; Müller, W.E.G. Effect of bioglass on growth and biomineralization of saos-2 cells in hydrogel after 3D cell bioprinting. PLoS ONE 2014, 9, e112497. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.H.; Ahn, S.H.; Yang, G.-H.; Kim, G.H. A MSCs-laden polycaprolactone/collagen scaffold for bone tissue regeneration. RSC Adv. 2016, 6, 6259–6265. [Google Scholar] [CrossRef]
- Daly, A.C.; Cunniffe, G.M.; Sathy, B.N.; Jeon, O.; Alsberg, E.; Kelly, D.J. 3D Bioprinting of Developmentally Inspired Templates for Whole Bone Organ Engineering. Adv. Healthc. Mater. 2016. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, R.O.; Buehler, M.J.; Hansma, P. Plasticity and toughness in bone. Phys. Today 2009, 62, 41–47. [Google Scholar] [CrossRef]
- Kaklamani, G.; Cheneler, D.; Grover, L.M.; Adams, M.J.; Bowen, J. Mechanical properties of alginate hydrogels manufactured using external gelation. J. Mech. Behav. Biomed. Mater. 2014, 36, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Binder, K.W.; Albanna, M.Z.; Dice, D.; Zhao, W.; Yoo, J.J.; Atala, A. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication 2013, 5, 015001. [Google Scholar] [CrossRef]
- Kundu, J.; Shim, J.-H.; Jang, J.; Kim, S.-W.; Cho, D.-W. An additive manufacturing-based PCL-alginate-chondrocyte bioprinted scaffold for cartilage tissue engineering. J. Tissue Eng. Regen. Med. 2015, 9, 1286–1297. [Google Scholar] [CrossRef] [PubMed]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martínez Ávila, H.; Hägg, D.; Gatenholm, P. 3D bioprinting human chondrocytes with nanocellulose-alginate bioink for cartilage tissue engineering applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Bakarich, S.E.; Gorkin, R.; Panhuis, M.; Spinks, G.M. Three-dimensional printing fiber reinforced hydrogel composites. ACS Appl. Mater. Interfaces 2014, 6, 15998–16006. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Öztürk, E.; Arlov, Ø.; Gatenholm, P.; Zenobi-Wong, M. Alginate Sulfate–Nanocellulose Bioinks for Cartilage Bioprinting Applications. Ann. Biomed. Eng. 2016, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Izadifar, Z.; Chang, T.; Kulyk, W.; Chen, X.; Eames, B.F. Analyzing biological performance of 3D-printed, cell-impregnated hybrid constructs for cartilage tissue engineering. Tissue Eng. C Methods 2016, 22, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Chimene, D.; Lennox, K.K.; Kaunas, R.R.; Gaharwar, A.K. Advanced bioinks for 3D printing: A materials science perspective. Ann. Biomed. Eng. 2016, 44, 2090–2102. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Dévora, J.I.; Zhang, B.; Reyna, D.; Shi, Z.-D.; Xu, T. High throughput miniature drug-screening platform using bioprinting technology. Biofabrication 2012, 4, 035001. [Google Scholar] [CrossRef] [PubMed]
- Khalil, S.; Sun, W. Bioprinting endothelial cells with alginate for 3D tissue constructs. J. Biomech. Eng. 2009, 131. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Iwanaga, S.; Henmi, C.; Arai, K.; Nishiyama, Y. Biomatrices and biomaterials for future developments of bioprinting and biofabrication. Biofabrication 2010, 2, 014110. [Google Scholar] [CrossRef] [PubMed]
- Song, S.-J.; Choi, J.; Park, Y.-D.; Hong, S.; Lee, J.J.; Ahn, C.B.; Choi, H.; Sun, K. Sodium Alginate Hydrogel-Based Bioprinting Using a Novel Multinozzle Bioprinting System. Artif. Organs 2011, 35, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Tomaskovic-Crook, E.; Lozano, R.; Chen, Y.; Kapsa, R.M.; Zhou, Q.; Wallace, G.G.; Crook, J.M. Functional 3D Neural Mini-Tissues from Printed Gel-Based Bioink and Human Neural Stem Cells. Adv. Healthc. Mater. 2016, 5, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Faulkner-Jones, A.; Fyfe, C.; Cornelissen, D.-J.; Gardner, J.; King, J.; Courtney, A.; Shu, W. Bioprinting of human pluripotent stem cells and their directed differentiation into hepatocyte-like cells for the generation of mini-livers in 3D. Biofabrication 2015, 7, 044102. [Google Scholar] [CrossRef]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015, 1, e1500758. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.; Xu, Y.; Chen, X.; Schreyer, D.J. Influence of mechanical properties of alginate-based substrates on the performance of Schwann cells in culture. J. Biomater. Sci. Polym. Ed. 2016, 27, 898–915. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yao, R.; Ouyang, L.; Ding, H.; Zhang, T.; Zhang, K.; Cheng, S.; Sun, W. Three-dimensional printing of Hela cells for cervical tumor model in vitro. Biofabrication 2014, 6, 035001. [Google Scholar] [CrossRef] [PubMed]
- Poldervaart, M.T.; Wang, H.; van der Stok, J.; Weinans, H.; Leeuwenburgh, S.C.G.; Oner, F.C.; Dhert, W.J.; Alblas, J. Sustained release of BMP-2 in bioprinted alginate for osteogenicity in mice and rats. PLoS ONE 2013, 8, e72610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.; Su, X.; Xu, Y.; Kong, B.; Sun, W.; Mi, S. Bioprinting three-dimensional cell-laden tissue constructs with controllable degradation. Sci. Rep. 2016, 6, 24474. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.Y.; Naficy, S.; Yue, Z.; Kapsa, R.; Quigley, A.; Moulton, S.E.; Wallace, G.G. Bio-ink properties and printability for extrusion printing living cells. Biomater. Sci. 2013, 1, 763–773. [Google Scholar] [CrossRef]



| 3D Bioprinting Application | Problem (of the Use of Alginate) | Solution | Reference |
|---|---|---|---|
| General | Immunogenicity (low cell grow support) | Use a low amount of d-mannuronic acid | [15] |
| General | Fast gelation needed | Use multivalent cations 1 | [16] |
| General | Slow degradation kinetics | Tune the weight percent | [24] |
| General | Slow degradation kinetics | Oxidation | [23,49] |
| Vascular tissue | Lack of channels transporting oxygen and nutrients to cells | Use coaxial printing nozzles | [28,29,31] |
| Bone | Poor mechanical properties | Combination with hydroxypatite | [33] |
| Bone | Poor mechanical properties | Combination with polycaprolactone | [34] |
| Bone | Poor adhesion properties | Addition of adhesion peptides (Arg-Gly-Asp) | [39] |
| Cartilage | Need of biomimetic ECM 2 | Combination with polycaprolactone 3D constructs | [42,47] |
| Cartilage | Need of biomimetic ECM 2 | Combination with nanofibrillated cellulose | [44] |
| Cartilage | Need of biomimetic ECM 2 | Combination with acrylamide | [45] |
| Cartilage | Low printability of alginate sulfate | Combination with nanocellulose | [46] |
| Cartilage | Low ECM 2 formation | Combination with polycaprolactone and growth factors (TGFβ) | [43] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Axpe, E.; Oyen, M.L. Applications of Alginate-Based Bioinks in 3D Bioprinting. Int. J. Mol. Sci. 2016, 17, 1976. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17121976
Axpe E, Oyen ML. Applications of Alginate-Based Bioinks in 3D Bioprinting. International Journal of Molecular Sciences. 2016; 17(12):1976. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17121976
Chicago/Turabian StyleAxpe, Eneko, and Michelle L. Oyen. 2016. "Applications of Alginate-Based Bioinks in 3D Bioprinting" International Journal of Molecular Sciences 17, no. 12: 1976. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms17121976