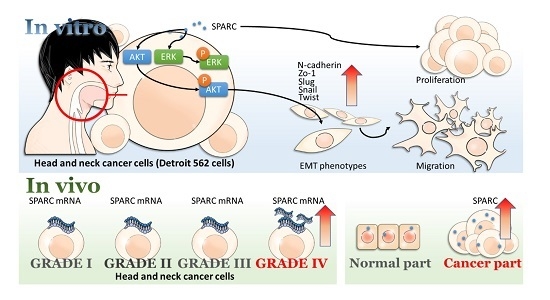
Secreted Protein Acidic and Rich in Cysteine (SPARC) Enhances Cell Proliferation, Migration, and Epithelial Mesenchymal Transition, and SPARC Expression is Associated with Tumor Grade in Head and Neck Cancer
Abstract
:1. Introduction
2. Results
2.1. Secreted Protein Acidic and Rich in Cysteine (SPARC) Treatment Enhances Cell Proliferation and Migration
2.2. SPARC Treatment Induces Epithelial Mesenchymal Transition (EMT) Phenotypes in Head and Neck Cancer Cells
2.3. Evaluation of SAPRC Treatment-Associated Kinases through Phosphor-Kinase Array
2.4. Investigation of SPARC Treatment Induced-Signaling Pathways
2.5. Evaluation of SPARC Expression in Clinical Samples
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Chemicals
4.3. Proliferation Assay
4.4. Migration Assay
4.5. Western Blot
4.6. Phospho-Kinase Array
4.7. cDNA Arrays Analysis
4.8. Human Tumor Samples
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Rezende, T.M.; de Souza Freire, M.; Franco, O.L. Head and neck cancer: Proteomic advances and biomarker achievements. Cancer 2010, 116, 4914–4925. [Google Scholar] [CrossRef] [PubMed]
- Kreimer, A.R.; Clifford, G.M.; Boyle, P.; Franceschi, S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: A systematic review. Cancer Epidemiol. Biomarkers Prev. 2005, 14, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Gilyoma, J.M.; Rambau, P.F.; Masalu, N.; Kayange, N.M.; Chalya, P.L. Head and neck cancers: A clinico-pathological profile and management challenges in a resource-limited setting. BMC Res. Notes 2015, 8, 772. [Google Scholar] [CrossRef] [PubMed]
- Eckert, A.W.; Wickenhauser, C.; Salins, P.C.; Kappler, M.; Bukur, J.; Seliger, B. Clinical relevance of the tumor microenvironment and immune escape of oral squamous cell carcinoma. J. Transl. Med. 2016, 14, 85. [Google Scholar] [CrossRef] [PubMed]
- Multhaupt, H.A.; Leitinger, B.; Gullberg, D.; Couchman, J.R. Extracellular matrix component signaling in cancer. Adv. Drug Deliv. Rev. 2016, 97, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, P.; Sage, E.H. Matricellular proteins: Extracellular modulators of cell function. Curr. Opin. Cell Biol. 2002, 14, 608–616. [Google Scholar] [CrossRef]
- Brekken, R.A.; Sage, E.H. Sparc, a matricellular protein: At the crossroads of cell-matrix communication. Matrix Biol. 2001, 19, 816–827. [Google Scholar] [CrossRef]
- Framson, P.E.; Sage, E.H. Sparc and tumor growth: Where the seed meets the soil? J. Cell. Biochem. 2004, 92, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Tang, L. Sparc in tumor pathophysiology and as a potential therapeutic target. Curr. Pharm. Des. 2014, 20, 6182–6190. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Yuan, P.; Du, F.; Hong, R.; Ding, X.; Shi, X.; Fan, Y.; Wang, J.; Luo, Y.; Ma, F.; et al. Sparc overexpression in primary tumors correlates with disease recurrence and overall survival in patients with triplenegative breast cancer. Oncotarget 2016, 7, 76628–76634. [Google Scholar] [CrossRef] [PubMed]
- Nagai, M.A.; Gerhard, R.; Fregnani, J.H.; Nonogaki, S.; Rierger, R.B.; Netto, M.M.; Soares, F.A. Prognostic value of ndrg1 and sparc protein expression in breast cancer patients. Breast Cancer Res. Treat. 2011, 126, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, J.; Zhao, Y.Y.; Jiang, W.; Xue, C.; Xu, F.; Zhao, H.Y.; Zhang, Y.; Zhao, L.P.; Hu, Z.H.; et al. Sparc expression and prognostic value in non-small cell lung cancer. Chin. J. Cancer 2012, 31, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.H.; Espinosa, I.; Gilks, C.B.; van de Rijn, M.; West, R.B. The fibromatosis signature defines a robust stromal response in breast carcinoma. Lab. Investig. 2008, 88, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Guttlein, L.N.; Benedetti, L.G.; Fresno, C.; Spallanzani, R.G.; Mansilla, S.F.; Rotondaro, C.; Raffo Iraolagoitia, X.L.; Salvatierra, E.; Bravo, A.I.; Fernandez, E.A.; et al. Predictive outcomes for HER2-enriched cancer using growth and metastasis signatures driven by SPARC. Mol. Cancer Res. 2017, 15, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Fukushima, N.; Maehara, N.; Matsubayashi, H.; Koopmann, J.; Su, G.H.; Hruban, R.H.; Goggins, M. SPARC/osteonectin is a frequent target for aberrant methylation in pancreatic adenocarcinoma and a mediator of tumor-stromal interactions. Oncogene 2003, 22, 5021–5030. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Polette, M.; Mestdagt, M.; Bindels, S.; Nawrocki-Raby, B.; Hunziker, W.; Foidart, J.M.; Birembaut, P.; Gilles, C. β-catenin and Zo-1: Shuttle molecules involved in tumor invasion-associated epithelial-mesenchymal transition processes. Cells Tissues Organs 2007, 185, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Giudici, C.; Raynal, N.; Wiedemann, H.; Cabral, W.A.; Marini, J.C.; Timpl, R.; Bachinger, H.P.; Farndale, R.W.; Sasaki, T.; Tenni, R. Mapping of SPARC/BM-40/osteonectin-binding sites on fibrillar collagens. J. Biol. Chem. 2008, 283, 19551–19560. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, A.D. The role of sparc in extracellular matrix assembly. J. Cell Commun. Signal. 2009, 3, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Barker, T.H.; Baneyx, G.; Cardo-Vila, M.; Workman, G.A.; Weaver, M.; Menon, P.M.; Dedhar, S.; Rempel, S.A.; Arap, W.; Pasqualini, R.; et al. SPARC regulates extracellular matrix organization through its modulation of integrin-linked kinase activity. J. Biol. Chem. 2005, 280, 36483–36493. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Sage, E.H. SPARC inhibits adipogenesis by its enhancement of β-catenin signaling. J. Biol. Chem. 2009, 284, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.L.; Alam, R.; Lemke, N.; Schultz, L.R.; Gutierrez, J.A.; Rempel, S.A. Pten augments SPARC suppression of proliferation and inhibits SPARC-induced migration by suppressing SHC-RAF-ERK and AKT signaling. Neuro Oncol. 2010, 12, 941–955. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Mizokami, A.; Kim, J.; Ofude, M.; Konaka, H.; Kadono, Y.; Kitagawa, Y.; Miwa, S.; Kumaki, M.; Keller, E.T.; et al. Exogenous sparc suppresses proliferation and migration of prostate cancer by interacting with integrin β1. Prostate 2013, 73, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Dedhar, S.; Kalluri, R.; Thompson, E.W. The epithelial-mesenchymal transition: New insights in signaling, development, and disease. J. Cell Biol. 2006, 172, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, C.S.; Van Tubergen, E.A.; Inglehart, R.C.; D’Silva, N.J. Biomarkers of epithelial-mesenchymal transition in squamous cell carcinoma. J. Dent. Res. 2013, 92, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, J.D.; Yan, L.J.; Zhao, X.P. PDGF-D/PDGFRβ promotes tongue squamous carcinoma cell (TSCC) progression via activating p38/AKT/ERK/EMT signal pathway. Biochem. Biophys. Res. Commun. 2016, 478, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Cheng, J.; Sun, G.; Wu, S.; Li, M.; Gao, Z.; Zhai, S.; Li, P.; Su, D.; Wang, X. P70s6k promotes IL-6-induced epithelial-mesenchymal transition and metastasis of head and neck squamous cell carcinoma. Oncotarget 2016, 7, 36539–36550. [Google Scholar] [CrossRef] [PubMed]
- Visciano, C.; Liotti, F.; Prevete, N.; Cali, G.; Franco, R.; Collina, F.; de Paulis, A.; Marone, G.; Santoro, M.; Melillo, R.M. Mast cells induce epithelial-to-mesenchymal transition and stem cell features in human thyroid cancer cells through an IL-8-AKT-Slug pathway. Oncogene 2015, 34, 5175–5186. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Hu, Z.; Jiang, Y.; Sun, R.; Chen, X.; Chu, H.; Zeng, M.; Sun, C. Interleukin-11 promotes epithelial-mesenchymal transition in anaplastic thyroid carcinoma cells through PI3K/AKT/GSK3β signaling pathway activation. Oncotarget 2016, 7, 59652–59663. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Gao, M.; Shen, Z.; Luo, B.; Li, R.; Jiang, X.; Ding, R.; Ha, Y.; Wang, Z.; Jie, W. Blocking PI3K/AKT signaling attenuates metastasis of nasopharyngeal carcinoma cells through induction of mesenchymal-epithelial reverting transition. Oncol. Rep. 2014, 32, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Nagashima, Y.; Baba, Y.; Kawano, T.; Furukawa, M.; Kubota, A.; Yanoma, S.; Imagawa-Ishiguro, Y.; Satake, K.; Taguchi, T.; et al. Expression of sparc in tongue carcinoma of stage II is associated with poor prognosis: An immunohistochemical study of 86 cases. Int. J. Mol. Med. 2005, 16, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Q.; Zhang, Q.; Liu, X.K.; Li, C.Q.; Guo, Z.M. Expression and clinical significance of SPARC in clinical stage II tongue squamous cell carcinoma. Chin. J. Cancer 2009, 28, 68–71. [Google Scholar]
- Wang, H.Y.; Li, Y.Y.; Shao, Q.; Hou, J.H.; Wang, F.; Cai, M.B.; Zeng, Y.X.; Shao, J.Y. Secreted protein acidic and rich in cysteine (SPARC) is associated with nasopharyngeal carcinoma metastasis and poor prognosis. J. Transl. Med. 2012, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Choi, P.; Jordan, C.D.; Mendez, E.; Houck, J.; Yueh, B.; Farwell, D.G.; Futran, N.; Chen, C. Examination of oral cancer biomarkers by tissue microarray analysis. Arch. Otolaryngol. Head Neck Surg. 2008, 134, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Aquino, G.; Sabatino, R.; Cantile, M.; Aversa, C.; Ionna, F.; Botti, G.; La Mantia, E.; Collina, F.; Malzone, G.; Pannone, G.; et al. Expression analysis of SPARC/osteonectin in oral squamous cell carcinoma patients: From saliva to surgical specimen. Biomed. Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Nagaraju, G.P.; Sharma, D. Anti-cancer role of sparc, an inhibitor of adipogenesis. Cancer Treat. Rev. 2011, 37, 559–566. [Google Scholar] [CrossRef] [PubMed]







© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-H.; Yen, M.-C.; Liao, S.-H.; Hsu, Y.-L.; Lai, C.-S.; Chang, K.-P.; Hsu, Y.-L. Secreted Protein Acidic and Rich in Cysteine (SPARC) Enhances Cell Proliferation, Migration, and Epithelial Mesenchymal Transition, and SPARC Expression is Associated with Tumor Grade in Head and Neck Cancer. Int. J. Mol. Sci. 2017, 18, 1556. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18071556
Chang C-H, Yen M-C, Liao S-H, Hsu Y-L, Lai C-S, Chang K-P, Hsu Y-L. Secreted Protein Acidic and Rich in Cysteine (SPARC) Enhances Cell Proliferation, Migration, and Epithelial Mesenchymal Transition, and SPARC Expression is Associated with Tumor Grade in Head and Neck Cancer. International Journal of Molecular Sciences. 2017; 18(7):1556. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18071556
Chicago/Turabian StyleChang, Chih-Hau, Meng-Chi Yen, Ssu-Hui Liao, Yu-Ling Hsu, Chung-Sheng Lai, Kao-Ping Chang, and Ya-Ling Hsu. 2017. "Secreted Protein Acidic and Rich in Cysteine (SPARC) Enhances Cell Proliferation, Migration, and Epithelial Mesenchymal Transition, and SPARC Expression is Associated with Tumor Grade in Head and Neck Cancer" International Journal of Molecular Sciences 18, no. 7: 1556. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18071556