Regulation of Aquaporin Functional Properties Mediated by the Antioxidant Effects of Natural Compounds
Abstract
:1. Introduction
2. Results
2.1. Compound Selection and Free Radical Scavenging (FRS) Activity
2.2. AQP1, 3, 8, 9, and 11 mRNA Are Expressed in HeLa Cells
2.3. AQP1, 3, 8, and 11, but Not AQP9, Protein Are Expressed in HeLa Cells
2.4. Effect of Antioxidant Compounds on the Water Permeability in the Presence and in the Absence of Oxidative Stress
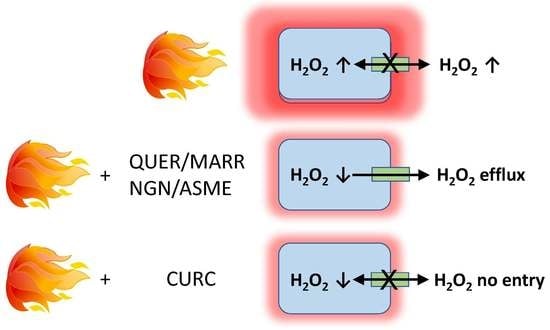
2.5. Effect of Antioxidant Compounds on the H2O2 Content in Heat-Stressed HeLa Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. RNA Isolation and RT-PCR
4.3. Immunoblotting
4.4. DPPH Assay
4.5. Water Permeability Measurements
4.6. Hydrogen Peroxide Measurements
4.7. Protein Content
4.8. Statistics
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| H2O2 | Hydrogen peroxide |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| NOX | NADPH oxidases |
| AQP | Aquaporin |
| CM-H2DCFDA | 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester |
| ASME | (R)-aloesaponol III-8 methyl ether |
| NRG | Naringenin |
| QUER | Quercetin |
| MARR | Marrubiin |
| CURC | Curcumin |
| FRS | Free radical scavenging |
| DPPH | α,α-diphenyl-β-picrylhydrazyl |
| SEM | Standard error mean |
References
- Agarwal, A.; Virk, G.; Ong, C.; du Plessis, S.S. Effect of oxidative stress on male reproduction. World J. Men’s Health 2014, 32, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Alfadda, A.A.; Sallam, R.M. Reactive oxygen species in health and disease. J. Biomed. Biotechnol. 2012, 2012, 936486. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Li, J.; Liu, Z.; Chuang, C.C.; Yang, W.; Zuo, L. Redox mechanism of reactive oxygen species in exercise. Front. Physiol. 2016, 7, 486. [Google Scholar] [CrossRef] [PubMed]
- Kakihana, T.; Nagata, K.; Sitia, R. Peroxides and peroxidases in the endoplasmic reticulum: Integrating redox homeostasis and oxidative folding. Antioxid. Redox Signal. 2012, 16, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Role of metabolic H2O2 generation: Redox signaling and oxidative stress. J. Biol. Chem. 2014, 289, 8735–8741. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Schjoerring, J.K.; Jahn, T.P. Membrane transport of hydrogen peroxide. Biochim. Biophys. Acta 2006, 1758, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Henzler, T.; Steudle, E. Transport and metabolic degradation of hydrogen peroxide in Chara corallina: Model calculations and measurements with the pressure probe suggest transport of H2O2 across water channels. J. Exp. Bot. 2000, 51, 2053–2066. [Google Scholar] [CrossRef] [PubMed]
- Laforenza, U.; Bottino, C.; Gastaldi, G. Mammalian aquaglyceroporin function in metabolism. Biochim. Biophys. Acta 2016, 1858, 1–11. [Google Scholar] [CrossRef] [PubMed]
- King, L.S.; Kozono, D.; Agre, P. From structure to disease: The evolving tale of aquaporin biology. Nat. Rev. Mol. Cell Biol. 2004, 5, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Benga, G. Foreword to the special issue on water channel proteins (aquaporins and relatives) in health and disease: 25 years after the discovery of the first water channel protein, later called aquaporin 1. Mol. Asp. Med. 2012, 33, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Møller, A.L.; Kristiansen, K.A.; Schulz, A.; Møller, I.M.; Schjoerring, J.K.; Jahn, T.P. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 2007, 282, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Chaumont, F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta 2014, 1840, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Medraño-Fernandez, I.; Bestetti, S.; Bertolotti, M.; Bienert, G.P.; Bottino, C.; Laforenza, U.; Rubartelli, A.; Sitia, R. Stress regulates Aquaporin-8 permeability to impact cell growth and survival. Antioxid. Redox Signal. 2016, 24, 1031–1044. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.W.; Dickinson, B.C.; Chang, C.J. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 15681–15686. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Chikuma, S.; Sugiyama, Y.; Kabashima, K.; Verkman, A.S.; Inoue, S.; Miyachi, Y. Chemokine-dependent T cell migration requires aquaporin-3-mediated hydrogen peroxide uptake. J. Exp. Med. 2012, 209, 1743–1752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, S.; Moniaga, C.S.; Nielsen, S.; Hara-Chikuma, M. Aquaporin-9 facilitates membrane transport of hydrogen peroxide in mammalian cells. Biochem. Biophys. Res. Commun. 2016, 471, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Thiagarajah, J.R.; Chang, J.; Goettel, J.A.; Verkman, A.S.; Lencer, W.I. Aquaporin-3 mediates hydrogen peroxide-dependent responses to environmental stress in colonic epithelia. Proc. Natl. Acad. Sci. USA 2017, 114, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Mósca, A.F.; Martins, A.P.; Nobre, T.; Prista, C.; Antunes, F.; Cipak Gasparovic, A.; Soveral, G. Rat Aquaporin-5 is pH-Gated induced by phosphorylation and is implicated in oxidative stress. Int. J. Mol. Sci. 2016, 17, 2090. [Google Scholar] [CrossRef] [PubMed]
- Almasalmeh, A.; Krenc, D.; Wu, B.; Beitz, E. Structural determinants of the hydrogen peroxide permeability of aquaporins. FEBS J. 2014, 281, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Atochina-Vasserman, E.N.; Biktasova, A.; Abramova, E.; Cheng, D.S.; Polosukhin, V.V.; Tanjore, H.; Takahashi, S.; Sonoda, H.; Foye, L.; Venkov, C.; et al. Aquaporin 11 insufficiency modulates kidney susceptibility to oxidative stress. Am. J. Physiol. Ren. Physiol. 2013, 304, 1295–1307. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Watanabe, S.; Satooka, H. Involvement of aquaporin-3 in epidermal growth factor receptor signaling via hydrogen peroxide transport in cancer cells. Biochem. Biophys. Res. Commun. 2016, 471, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Satooka, H.; Hara-Chikuma, M. Aquaporin-3 controls breast cancer cell migration by regulating hydrogen peroxide transport and its downstream cell signaling. Mol. Cell. Biol. 2016, 36, 1206–1218. [Google Scholar] [CrossRef] [PubMed]
- Bertolotti, M.; Bestetti, S.; García-Manteiga, J.M.; Medraño-Fernandez, I.; Dal Mas, A.; Malosio, M.L.; Sitia, R. Tyrosine kinase signal modulation: A matter of H2O2 membrane permeability? Antioxid. Redox Signal. 2013, 19, 1447–1451. [Google Scholar] [CrossRef] [PubMed]
- Laforenza, U.; Pellavio, G.; Marchetti, A.L.; Omes, C.; Todaro, F.; Gastaldi, G. Aquaporin-mediated water and hydrogen peroxide transport is involved in normal human spermatozoa functioning. Int. J. Mol. Sci. 2016, 18, 66. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S.; Anderson, M.O.; Papadopoulos, M.C. Aquaporins: Important but elusive drug targets. Nat. Rev. Drug Discov. 2014, 13, 259–277. [Google Scholar] [CrossRef] [PubMed]
- Beitz, E.; Golldack, A.; Rothert, M.; von Bülow, J. Challenges and achievements in the therapeutic modulation of aquaporin functionality. Pharmacol. Ther. 2015, 155, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Gaggeri, R.; Rossi, D.; Collina, S.; Mannucci, B.; Baierl, M.; Juza, M. Quick development of an analytical enantioselective high performance liquid chromatography separation and preparative scale-up for the flavonoid Naringenin. J. Chromatogr. A 2011, 1218, 5414–5422. [Google Scholar] [CrossRef] [PubMed]
- Gaggeri, R.; Rossi, D.; Christodoulou, M.S.; Passarella, D.; Leoni, F.; Azzolina, O.; Collina, S. Chiral flavanones from amygdalus lycioides spach: Structural elucidation and identification of tnfalpha inhibitors by bioactivity-guided fractionation. Molecules 2012, 17, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Ademosun, A.O.; Ogunsuyi, O.B. Quercetin and its role in chronic diseases. Adv. Exp. Med. Biol. 2016, 929, 377–387. [Google Scholar] [PubMed]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sun, C.; Mao, L.; Ma, P.; Liu, F.; Yang, J.; Gao, Y. The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- Haghi, A.; Azimi, H.; Rahimi, R. A Comprehensive review on pharmacotherapeutics of three phytochemicals, curcumin, quercetin, and allicin, in the treatment of gastric cancer. J. Gastrointest. Cancer 2017, 48, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Rani, N.; Bharti, S.; Krishnamurthy, B.; Bhatia, J.; Sharma, C.; Kamal, M.A.; Ojha, S.; Arya, D.S. Pharmacological properties and therapeutic potential of naringenin: A citrus flavonoid of pharmaceutical promise. Curr. Pharm. Des. 2016, 22, 4341–4359. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Jin, L.; Han, X.; Song, Z.; Zhang, H.; Liang, W. Naringenin: A potential immunomodulator for inhibiting lung fibrosis and metastasis. Cancer Res. 2009, 69, 3205–3212. [Google Scholar] [CrossRef] [PubMed]
- Galati, G.; Sabzevari, O.; Wilson, J.X.; O’Brien, P.J. Prooxidant activity and cellular effects of the phenoxyl radicals of dietary flavonoids and other polyphenolics. Toxicology 2002, 177, 91–104. [Google Scholar] [CrossRef]
- Bodet, C.; La, V.D.; Epifano, F.; Grenier, D. Naringenin has anti-inflammatory properties in macrophage and ex vivo human whole-blood models. J. Periodontal Res. 2008, 43, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Shinkaruk, S.; Lamothe, V.; Schmitter, J.M.; Manach, C.; Morand, C.; Berard, A.; Bennetau, B.; Bennetau-Pelissero, C. Development and validation of two new sensitive ELISAs for Hesperetin and Naringenin in biological fluids. Food Chem. 2010, 118, 472–481. [Google Scholar] [CrossRef]
- Rossi, D.; Ahmed, K.M.; Gaggeri, R.; Della Volpe, S.; Maggi, L.; Mazzeo, G.; Longhi, G.; Abbate, S.; Corana, F.; Martino, E.; et al. (R)-(−)-Aloesaponol III 8-Methyl Ether from Eremurus persicus: A Novel Compound against Leishmaniosis. Molecules 2017, 22, 519. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Ahmadi, Y.; Teymouri, M.; Johnston, T.P.; Sahebkar, A. Curcumin as a potential candidate for treating hyperlipidemia: A review of cellular and metabolic mechanisms. J. Cell. Physiol. 2018, 233, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.F.; Silva, A.M.F.; Carvalho, A.A. The use of antioxidant agents for chemotherapy-induced peripheral neuropathy treatment in animal models. Clin. Exp. Pharmacol. Physiol. 2017, 44, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Adiwidjaja, J.; McLachlan, A.J.; Boddy, A.V. Curcumin as a clinically-promising anti-cancer agent: Pharmacokinetics and drug interactions. Expert Opin. Drug Metab. Toxicol. 2017, 13, 953–972. [Google Scholar] [CrossRef] [PubMed]
- Beukes, N.; Levendal, R.A.; Frost, C.L. Selected terpenoids from medicinal plants modulate endoplasmic reticulum stress in metabolic disorders. J. Pharm. Pharmacol. 2014, 66, 1505–1525. [Google Scholar] [CrossRef] [PubMed]
- Popoola, O.K.; Elbagory, A.M.; Ameer, F.; Hussein, A.A. Marrubiin. Molecules 2013, 18, 9049–9060. [Google Scholar] [CrossRef] [PubMed]
- Amri, B.; Ben Kaab, S.; Gouia, H.; Martino, E.; Collina, S.; Ben Kaâb, L.B. Copper-induced changes in nutrient uptake, enzymatic and non-enzymatic antioxidant systems in horehound (Marrubium vulgare L.). Bot. Sci. 2017, 95, 565–575. [Google Scholar] [CrossRef]
- Nguyen, P.D.; Sayagh, C.; Borie, N.; Lavaud, C. Anti-radical flavonol glycosides from the aerial parts of Cleome chelidonii L.f. Phytochemistry 2017, 142, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, S.W.; Moseley, P.L.; Buettner, G.R. Increased flux of free radicals in cells subjected to hyperthermia: Detection by electron paramagnetic resonance spin trapping. FEBS Lett. 1998, 431, 285–286. [Google Scholar] [CrossRef]
- Cataldo, I.; Maggio, A.; Gena, P.; de Bari, O.; Tamma, G.; Portincasa, P.; Calamita, G. Modulation of Aquaporins by dietary patterns and plant bioactive compounds. Curr. Med. Chem. 2017. [Google Scholar] [CrossRef]
- Kumar, B.; Gupta, S.K.; Nag, T.C.; Srivastava, S.; Saxena, R.; Jha, K.A.; Srinivasan, B.P. Retinal neuroprotective effects of quercetin in streptozotocin-induced diabetic rats. Exp. Eye Res. 2014, 125, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Inoue, H.; Mishima, K.; Ide, F.; Nakayama, R.; Hasaka, A.; Ryo, K.; Ito, Y.; Sakurai, T.; Hasegawa, Y.; et al. Evaluation of the effects of quercetin on damaged salivary secretion. PLoS ONE 2015, 10, e0116008. [Google Scholar] [CrossRef] [PubMed]
- Tradtrantip, L.; Zhang, H.; Anderson, M.O.; Saadoun, S.; Phuan, P.W.; Papadopoulos, M.C.; Bennett, J.L.; Verkman, A.S. Small-molecule inhibitors of NMO-IgG binding to aquaporin-4 reduce astrocyte cytotoxicity in neuromyelitis optica. FASEB J. 2012, 26, 2197–2208. [Google Scholar] [CrossRef] [PubMed]
- Mangiatordi, G.F.; Alberga, D.; Siragusa, L.; Goracci, L.; Lattanzi, G.; Nicolotti, O. Challenging AQP4 druggability for NMO-IgG antibody binding using molecular dynamics and molecular interaction fields. Biochim. Biophys. Acta 2015, 1848, 1462–1471. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Zhang, B.; Duan, D.; Wu, J.; Fang, J. Curcumin targeting the thioredoxin system elevates oxidative stress in HeLa cells. Toxicol. Appl. Pharmacol. 2012, 262, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Fiorentini, D.; Zambonin, L.; Dalla Sega, F.V.; Hrelia, S. Polyphenols as modulators of aquaporin family in health and disease. Oxid. Med. Cell. Longev. 2015, 2015, 196914. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Yi, J.; Ye, G.; Zheng, Y.; Song, Z.; Yang, Y.; Song, Y.; Wang, Z.; Bao, Q. Effects of curcumin on levels of nitric oxide synthase and AQP-4 in a rat model of hypoxia-ischemic brain damage. Brain Res. 2012, 1475, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Laird, M.D.; Sukumari-Ramesh, S.; Swift, A.E.; Meiler, S.E.; Vender, J.R.; Dhandapani, K.M. Curcumin attenuates cerebral edema following traumatic brain injury in mice: A possible role for aquaporin-4? J. Neurochem. 2010, 113, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.S.; Fan, Y.Y.; Ye, G.; Li, J.; Feng, X.P.; Lin, K.; Dong, M.; Wang, Z. Curcumin alleviates brain edema by lowering AQP4 expression levels in a rat model of hypoxia-hypercapnia-induced brain damage. Exp. Ther. Med. 2016, 11, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Zu, J.; Wang, Y.; Xu, G.; Zhuang, J.; Gong, H.; Yan, J. Curcumin improves the recovery of motor function and reduces spinal cord edema in a rat acute spinal cord injury model by inhibiting the JAK/STAT signaling pathway. Acta Histochem. 2014, 116, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.F.; Cui, Z.W.; Zhong, Z.H.; Sun, Y.H.; Sun, Q.F.; Yang, G.Y.; Bian, L.G. Curcumin attenuates brain edema in mice with intracerebral hemorrhage through inhibition of AQP4 and AQP9 expression. Acta Pharmacol. Sin. 2015, 36, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Nabiuni, M.; Nazari, Z.; Safaeinejad, Z.; Delfan, B.; Miyan, J.A. Curcumin downregulates aquaporin-1 expression in cultured rat choroid plexus cells. J. Med. Food 2013, 16, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Cao, C.; Lu, S.; Kivlin, R.; Amaral, A.; Kouttab, N.; Yang, H.; Chu, W.; Bi, Z.; Di, W.; Wan, Y. Curcumin attenuates EGF-induced AQP3 up-regulation and cell migration in human ovarian cancer cells. Cancer Chemother. Pharmacol. 2008, 62, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Laforenza, U.; Gastaldi, G.; Grazioli, M.; Cova, E.; Tritto, S.; Faelli, A.; Calamita, G.; Ventura, U. Expression and immunolocalization of aquaporin-7 in rat gastrointestinal tract. Biol. Cell 2005, 97, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Laforenza, U.; Scaffino, M.F.; Gastaldi, G. Aquaporin-10 represents an alternative pathway for glycerol efflux from human adipocytes. PLoS ONE 2013, 8, e54474. [Google Scholar] [CrossRef] [PubMed]
- Laforenza, U. Water channel proteins in the gastrointestinal tract. Mol. Aspects Med. 2012, 33, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Laforenza, U.; Cova, E.; Gastaldi, G.; Tritto, S.; Grazioli, M.; LaRusso, N.F.; Splinter, P.L.; D’Adamo, P.; Tosco, M.; Ventura, U. Aquaporin-8 is involved in water transport in isolated superficial colonocytes from rat proximal colon. J. Nutr. 2005, 135, 2329–2336. [Google Scholar] [PubMed]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]








| Compound | Chemical Class | Natural Sources | Biological Activity | References |
|---|---|---|---|---|
| QUER | flavonoid | caper, black chokeberry, onion, tomato and lettuce | antioxidant, anti-inflammatory, anti-obesity, antiviral, antibacterial, anti-cancer and others | [30,31,32,33] |
| NRG | flavanone | Citrus fruits, tomato, pears, Amygdalus lycioides | Antoxidant, anti-inflammatory, antimicrobical, anti-cancer and immunomodulating activities | [34,35,36,37,38] |
| ASME | tetrahydroanthracene | Eremurus persicus; Aloe saponaria; Kniphonia foliosa; Eremurus chinensis | Antileishmanial activity | [39] |
| CURC | phenol | Curcuma longa, Curcuma species | Antioxidant, anti-inflammatory, antinociceptive, anticancer, immunomodulating properties and others | [33,40,41,42] |
| MARR | terpenoid | Marrubium vulgare, M. spicies, Phlomis bracteosa, Leonotis nepetifolia | antinociceptive, antioxidant, cardioprotective, vasorelaxant, gastroprotective, antispasmodic, immunomodulating, antioedematogenic, analgesic, antidiabetic properties | [43,44] |
| Gene | Primer Sequences | Size (bp) | TM (°C) | Accession Number | |
|---|---|---|---|---|---|
| AQP1 | Forward | 5′-TTAACCCTGCTCGGTCCTTT-3′ | 229 | 60 | NM_198098 |
| Reverse | 5′-TTCATCTCCACCCTGGAGTT-3′ | ||||
| AQP2 | Forward | 5′-CCACACTCCTCTTCGTCTTCTT-3′ | 552 | 60 | NM_000486 |
| Reverse | 5′-CCCAGTGGTCATCAAATTTGCC-3′ | ||||
| AQP3 | Forward | 5′-TTTGCTACCTACCCCTCTGG-3′ | 414 | 60 | NM_004925 |
| Reverse | 5′-GGCCAGCTTCACATTCTCTT-3′ | ||||
| AQP4 | Forward | 5′-GGTCTCCTGGTTGAGTTGAT-3′ | 346 | 60 | NM_004028 |
| Reverse | 5′-TTGTTTGCTGGGCAGCTTTG-3′ | ||||
| AQP5 | Forward | 5′-GAGCTGATTCTGACCTTCCA-3′ | 333 | 58 | NM_001651 |
| Reverse | 5′-AGGCTCATACGTGCCTTTGA-3′ | ||||
| AQP6 | Forward | 5′-GGGCTGTATGTGTTCTTTGG-3′ | 326 | 60 | NM_001652 |
| Reverse | 5′-TGGCCAGTTGAGACACTGTT-3′ | ||||
| AQP7 | Hs_AQP7_1_SG QuantiTect Primer Assay QT00067592 * | 139 | 60 | NM_001170 | |
| AQP8 | Forward | 5′-AGGAGAGGTTCTGGAATGCA-3′ | 282 | 60 | NM_001169 |
| Reverse | 5′-AGTGGAAGTTCCAGTGGTTG-3′ | ||||
| AQP9 | Forward | 5′-AAGCTTCAAGCAGAGACTGG-3′ | 432 | 60 | NM_020980 |
| Reverse | 5′-GGAGCTGGGTATGTTGCAAA-3′ | ||||
| AQP10 | Forward | 5′-CCCTATCTGTCCCTGAACAA-3′ | 554 | 60 | NM_080429 |
| Reverse | 5′-CCAGGTTCTGGCACATTAAC-3′ | ||||
| AQP11 | Forward | 5′-GGCTGCACTCATCACCTTTT-3′ | 141 | 58 | NM_173039 |
| Reverse | 5′-GGAGCCAGCCAGTATACTAT-3′ | ||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellavio, G.; Rui, M.; Caliogna, L.; Martino, E.; Gastaldi, G.; Collina, S.; Laforenza, U. Regulation of Aquaporin Functional Properties Mediated by the Antioxidant Effects of Natural Compounds. Int. J. Mol. Sci. 2017, 18, 2665. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18122665
Pellavio G, Rui M, Caliogna L, Martino E, Gastaldi G, Collina S, Laforenza U. Regulation of Aquaporin Functional Properties Mediated by the Antioxidant Effects of Natural Compounds. International Journal of Molecular Sciences. 2017; 18(12):2665. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18122665
Chicago/Turabian StylePellavio, Giorgia, Marta Rui, Laura Caliogna, Emanuela Martino, Giulia Gastaldi, Simona Collina, and Umberto Laforenza. 2017. "Regulation of Aquaporin Functional Properties Mediated by the Antioxidant Effects of Natural Compounds" International Journal of Molecular Sciences 18, no. 12: 2665. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms18122665