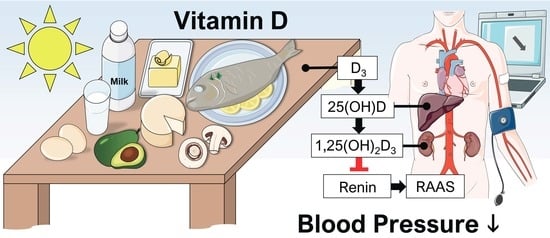
The Impact of Vitamin D in the Treatment of Essential Hypertension
Abstract
:1. Introduction
2. Arterial Hypertension
2.1. Definition, Causes and Risks
2.2. Management of Hypertension
3. Vitamin D
3.1. The Vitamin D Receptor (VDR)
3.2. Vitamin D Status
3.3. Suppression of Renin Production
4. Effects of Vitamin D on the Local Renin-Angiotensin System (RAS)
5. Vitamin D and Essential Hypertension
6. Discussion
7. Conclusions and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 1,25(OH)2D3 | Calcitriol |
| 25(OH)D | Calcidiol |
| 95%-CI | 95% confidence interval |
| AC | Adenylate cyclase |
| ACE | Angiotensin-converting enzyme |
| ACEi | Angiotensin-converting enzyme inhibitor |
| ANG | Angiotensin |
| ARB | Angiotensin II receptor blocker |
| AT1/AT2 | Angiotensin receptor type 1 or 2 |
| BMI | Body mass index |
| BP | Blood pressure |
| cAMP | cyclic adenosine monophosphate |
| CBP | CREB-binding protein |
| CO | Cardiac output |
| CRE | cAMP response element |
| CREB | cAMP-dependent response element binding protein |
| CVD | Cardiovascular disease |
| CYP | Cytochrome P450 |
| DBP | Vitamin D binding protein |
| EH | Essential hypertension |
| HR | Heart rate |
| IU | International units |
| MAP | Mean arterial pressure |
| mRNA | messenger ribonucleic acid |
| NHANES | National Health and Nutrition Examination Survey |
| OR | Odds-ratio |
| PKA | Protein kinase A |
| RAAS | Renin-angiotensin-aldosterone system |
| RCT | Randomized controlled trial |
| RR | Relative risk |
| RXR | Retinoid X receptor |
| SBP | Systolic blood pressure |
| TPR | Total periphery resistance |
| VDR | Vitamin D receptor |
| WHO | World Health Organization |
References
- A Global Brief on Hypertension. Available online: http://apps.who.int/iris/bitstream/10665/79059/1/WHO_DCO_WHD_2013.2_eng.pdf (accessed on 30 October 2017).
- Ke, L.; Mason, R.S.; Kariuki, M.; Mpofu, E.; Brock, K.E. Vitamin D status and hypertension: A review. Integr. Blood Press. Control. 2015, 8, 13–35. [Google Scholar] [PubMed]
- Mehta, V.; Agarwal, S. Does Vitamin D Deficiency Lead to Hypertension? Cureus 2017, 9, e1038. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Nie, X.L.; Wu, S.; Cai, J. Vitamin D and hypertension: Prospective study and meta-analysis. PLoS ONE 2017, 12, e0174298. [Google Scholar] [CrossRef] [PubMed]
- Geleijnse, J.M. Vitamin D and the Prevention of Hypertension and Cardiovascular Diseases: A Review of the Current Evidence. Am. J. Hypertens. 2011, 24, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A.; Forman, J.P. Vitamin D and Hypertension: Current Evidence and Future Directions. Hypertension 2010, 56, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Delmi, M.; Rapin, C.H.; Bengoa, J.M.; Delmas, P.D.; Vasey, H.; Bonjour, J.P. Dietary supplementation in elderly patients with fractured neck of the femur. Lancet 1990, 335, 1013–1016. [Google Scholar] [CrossRef]
- Shamardl, H.A.; El-Ashmony, S.M.; Kamel, H.F.; Fatani, S.H. Potenial Cardiovascular and Renal Protective Effects of Vitamin D and Coenzyme Q10 in l-NAME-Induced Hypertensive Rats. Am. J. Med. Sci. 2017, 354, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redon, J.; Zanchetti, A.; Bohm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur. Heart J. 2013, 34, 2159–2219. [Google Scholar] [PubMed]
- Kronborg, C.N.; Hallas, J.; Jacobsen, I.A. Prevalence, awareness, and control of arterial hypertension in Denmark. J. Am. Soc. Hypertens. 2009, 3, 19.e2–24.e2. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2017. [Google Scholar] [CrossRef]
- Hill, L.K.; Sollers Iii, J.J.; Thayer, J.F. Resistance reconstructed estimation of total peripheral resistance from computationally derived cardiac output—Biomed 2013. Biomed. Sci. Instrum. 2013, 49, 216–223. [Google Scholar] [PubMed]
- Laurent, S.; Boutouyrie, P. The structural factor of hypertension: Large and small artery alterations. Circ. Res. 2015, 116, 1007–1021. [Google Scholar] [CrossRef] [PubMed]
- Lewington, S.; Clarke, R.; Qizilbash, N.; Peto, R.; Collins, R. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar] [CrossRef]
- Saklayen, M.G.; Deshpande, N.V. Timeline of History of Hypertension Treatment. Front. Cardiovasc. Med. 2016, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.D. How do thiazide and thiazide-like diuretics lower blood pressure? J. Renin Angiotensin Aldoster. Syst. 2004, 5, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Brøsen, K.; Simonsen, U.; Kampmann, J.P.; Thirstrup, S. Basal Og Klinisk Farmakologi, 5th ed.; FADL’s Forlag: Copenhagen, Denmark, 2014; ISBN 978-87-7749-683-7. [Google Scholar]
- Striessnig, J.; Ortner, N.J.; Pinggera, A. Pharmacology of L-type Calcium Channels: Novel Drugs for Old Targets? Curr. Mol. Pharmacol. 2015, 8, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.P.; Giles, T.D.; Sowers, J.R. Evolving mechanisms of action of beta blockers: Focus on nebivolol. J. Cardiovasc. Pharmacol. 2009, 54, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Van Vark, L.C.; Bertrand, M.; Akkerhuis, K.M.; Brugts, J.J.; Fox, K.; Mourad, J.J.; Boersma, E. Angiotensin-converting enzyme inhibitors reduce mortality in hypertension: A meta-analysis of randomized clinical trials of renin-angiotensin-aldosterone system inhibitors involving 158,998 patients. Eur. Heart J. 2012, 33, 2088–2097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abramov, D.; Carson, P.E. The role of angiotensin receptor blockers in reducing the risk of cardiovascular disease. J. Renin Angiotensin Aldoster. Syst. 2012, 13, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Pool, J.L. Direct renin inhibition: Focus on aliskiren. J. Manag. Care Pharm. 2007, 13, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the immune system. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Brumbaugh, P.F.; Haussler, M.R. Nuclear and cytoplasmic binding components for vitamin D metabolites. Life Sci. 1975, 16, 353–362. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.; DeLuca, H.F. Where is the vitamin D receptor? Arch. Biochem. Biophys. 2012, 523, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Farrell, C.J.; Herrmann, M. Determination of vitamin D and its metabolites. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 675–688. [Google Scholar] [CrossRef] [PubMed]
- Razzaque, M.S. Can adverse effects of excessive vitamin D supplementation occur without developing hypervitaminosis D? J. Steroid Biochem. Mol. Biol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Kong, J.; Wei, M.; Chen, Z.F.; Liu, S.Q.; Cao, L.P. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J. Clin. Investig. 2002, 110, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Pan, W.; Kong, J.; Zheng, W.; Szeto, F.L.; Wong, K.E.; Cohen, R.; Klopot, A.; Zhang, Z.; Li, Y.C. 1,25-dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cyclic AMP response element in the renin gene promoter. J. Biol. Chem. 2007, 282, 29821–29830. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Qiao, G.; Zhang, Z.; Liu, S.Q.; Li, Y.C. Targeted vitamin D receptor expression in juxtaglomerular cells suppresses renin expression independent of parathyroid hormone and calcium. Kidney Int. 2008, 74, 1577–1581. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Kong, J.; Chen, S.; Cao, L.P.; Qiao, G.; Zheng, W.; Liu, W.; Li, X.; Gardner, D.G.; Li, Y.C. Cardiac hypertrophy in vitamin D receptor knockout mice: Role of the systemic and cardiac renin-angiotensin systems. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E125–E132. [Google Scholar] [CrossRef] [PubMed]
- Bruckschlegel, G.; Holmer, S.R.; Jandeleit, K.; Grimm, D.; Muders, F.; Kromer, E.P.; Riegger, G.A.; Schunkert, H. Blockade of the renin-angiotensin system in cardiac pressure-overload hypertrophy in rats. Hypertension 1995, 25, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Grimm, D.; Jabusch, H.C.; Kossmehl, P.; Huber, M.; Fredersdorf, S.; Griese, D.P.; Kramer, B.K.; Kromer, E.P. Experimental diabetes and left ventricular hypertrophy: Effects of beta-receptor blockade. Cardiovasc. Pathol. 2002, 11, 229–237. [Google Scholar] [CrossRef]
- Jurkovicova, D.; Dobesova, Z.; Kunes, J.; Krizanova, O. Different expression of renin-angiotensin system components in hearts of normotensive and hypertensive rats. Physiol. Res. 2001, 50, 35–42. [Google Scholar] [PubMed]
- Rothermund, L.; Kreutz, R.; Kossmehl, P.; Fredersdorf, S.; Shakibaei, M.; Schulze-Tanzil, G.; Paul, M.; Grimm, D. Early onset of chondroitin sulfate and osteopontin expression in angiotensin II-dependent left ventricular hypertrophy. Am. J. Hypertens. 2002, 15, 644–652. [Google Scholar] [CrossRef]
- Potter, D.D.; Sobey, C.G.; Tompkins, P.K.; Rossen, J.D.; Heistad, D.D. Evidence that macrophages in atherosclerotic lesions contain angiotensin II. Circulation 1998, 98, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Szeto, F.L.; Reardon, C.A.; Yoon, D.; Wang, Y.; Wong, K.E.; Chen, Y.; Kong, J.; Liu, S.Q.; Thadhani, R.; Getz, G.S.; et al. Vitamin D receptor signaling inhibits atherosclerosis in mice. Mol. Endocrinol. 2012, 26, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Carey, R.M.; Siragy, H.M. The intrarenal renin-angiotensin system and diabetic nephropathy. Trends Endocrinol. Metab. 2003, 14, 274–281. [Google Scholar] [CrossRef]
- Wang, J.; Chen, L.; Chen, B.; Meliton, A.; Liu, S.Q.; Shi, Y.; Liu, T.; Deb, D.K.; Solway, J.; Li, Y.C. Chronic Activation of the Renin-Angiotensin System Induces Lung Fibrosis. Sci. Rep. 2015, 5, 15561. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, T.; Yao, L.; Xing, Y.; Zhao, X.; Fu, J.; Xue, X. Chronic vitamin D deficiency induces lung fibrosis through activation of the renin-angiotensin system. Sci. Rep. 2017, 7, 3312. [Google Scholar] [CrossRef] [PubMed]
- Kalupahana, N.S.; Moustaid-Moussa, N. The renin-angiotensin system: A link between obesity, inflammation and insulin resistance. Obes. Rev. 2012, 13, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Ma, C.; Shuai, B.; Yang, Y. Effects of 1,25-dihydroxyvitamin D3 on the local bone renin-angiotensin system in a murine model of glucocorticoid-induced osteoporosis. Exp. Ther. Med. 2017, 13, 3297–3304. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Li, Y.C.; Boucher, B.J.; Leung, P.S. A novel role for vitamin D: Modulation of expression and function of the local renin-angiotensin system in mouse pancreatic islets. Diabetologia 2011, 54, 2077–2081. [Google Scholar] [CrossRef] [PubMed]
- Chyba, M.M.; Washington, L.R. Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–1994. Series 1: Programs and collection procedures. Vital. Health Stat 1 1994, 32, 1–407. [Google Scholar]
- Martins, D.; Wolf, M.; Pan, D.; Zadshir, A.; Tareen, N.; Thadhani, R.; Felsenfeld, A.; Levine, B.; Mehrotra, R.; Norris, K. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: Data from the Third National Health and Nutrition Examination Survey. Arch. Intern. Med. 2007, 167, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Scragg, R.; Sowers, M.; Bell, C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. Am. J. Hypertens. 2007, 20, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Apekey, T.A.; Steur, M. Vitamin D and risk of future hypertension: Meta-analysis of 283,537 participants. Eur. J. Epidemiol. 2013, 28, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Mohr, S.B.; Garland, C.F.; Gorham, E.D.; Garland, F.C. The association between ultraviolet B irradiance, vitamin D status and incidence rates of type 1 diabetes in 51 regions worldwide. Diabetologia 2008, 51, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Gaksch, M.; Kienreich, K.; Grubler, M.; Verheyen, N.; Fahrleitner-Pammer, A.; Treiber, G.; Drechsler, C.; Obermayer-Pietsch, B.; Schwetz, V.; et al. Effects of vitamin D on blood pressure and cardiovascular risk factors: A randomized controlled trial. Hypertension 2015, 65, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.; Song, Y.; Dusek, J.; Plotnikoff, G.; Sabatine, M.S.; Cheng, S.; Valcour, A.; Swales, H.; Taylor, B.; Carney, E.; et al. Vitamin D therapy in individuals with prehypertension or hypertension: The DAYLIGHT trial. Circulation 2015, 131, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Larsen, T.; Mose, F.H.; Bech, J.N.; Hansen, A.B.; Pedersen, E.B. Effect of cholecalciferol supplementation during winter months in patients with hypertension: A randomized, placebo-controlled trial. Am. J. Hypertens. 2012, 25, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- McGreevy, C.; Barry, M.; Davenport, C.; Byrne, B.; Donaghy, C.; Collier, G.; Tormey, W.; Smith, D.; Bennett, K.; Williams, D. The effect of vitamin D supplementation on arterial stiffness in an elderly community-based population. J. Am. Soc. Hypertens. 2015, 9, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Witham, M.D.; Ireland, S.; Houston, J.G.; Gandy, S.J.; Waugh, S.; Macdonald, T.M.; Mackenzie, I.S.; Struthers, A.D. Vitamin D therapy to reduce blood pressure and left ventricular hypertrophy in resistant hypertension: Randomized, controlled trial. Hypertension 2014, 63, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Witham, M.D.; Price, R.J.; Struthers, A.D.; Donnan, P.T.; Messow, C.M.; Ford, I.; McMurdo, M.E. Cholecalciferol treatment to reduce blood pressure in older patients with isolated systolic hypertension: The VitDISH randomized controlled trial. JAMA Intern. Med. 2013, 173, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.R.; Liu, Z.Y.; Shi, Y.; Yin, D.W.; Wang, H.; Sha, Y.; Chen, Y.D. Vitamin D and nifedipine in the treatment of Chinese patients with grades I-II essential hypertension: A randomized placebo-controlled trial. Atherosclerosis 2014, 235, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari-Khosravi, H.; Loloei, S.; Mirjalili, M.R.; Barzegar, K. The effect of vitamin D supplementation on blood pressure in patients with elevated blood pressure and vitamin D deficiency: A randomized, double-blind, placebo-controlled trial. Blood Press. Monit. 2015, 20, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Beveridge, L.A.; Struthers, A.D.; Khan, F.; Jorde, R.; Scragg, R.; Macdonald, H.M.; Alvarez, J.A.; Boxer, R.S.; Dalbeni, A.; Gepner, A.D.; et al. Effect of Vitamin D Supplementation on Blood Pressure: A Systematic Review and Meta-analysis Incorporating Individual Patient Data. JAMA Intern. Med. 2015, 175, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Franczyk, A.; Stolarz-Skrzypek, K.; Wesolowska, A.; Czarnecka, D. Vitamin D and vitamin D receptor activators in treatment of hypertension and cardiovascular disease. Cardiovasc. Hematol. Disord. Drug Targets 2014, 14, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, C.; Romagnoli, E.; Pepe, J.; Russo, S.; Carlucci, L.; Piemonte, S.; Nieddu, L.; McMahon, D.J.; Singh, R.; Minisola, S. Long-term bioavailability after a single oral or intramuscular administration of 600,000 IU of ergocalciferol or cholecalciferol: Implications for treatment and prophylaxis. J. Clin. Endocrinol. Metab. 2013, 98, 2709–2715. [Google Scholar] [CrossRef] [PubMed]



| Classification | SBP (mmHg) | Header | DBP (mmHg) |
|---|---|---|---|
| Normal | <120 | and | <80 |
| Elevated | 120–129 | and | <80 |
| Hypertension, Stage 1 | 130–139 | or | 80–89 |
| Hypertension, Stage 2 | ≥140 | or | ≥90 |
| Recommendation | Children and Adolescents | Adults |
|---|---|---|
| The optimal concentration of 25(OH)D in plasma | 20–60 ng/mL ≈ 50–150 nM | 30–80 ng/mL ≈ 75–200 nM |
| Supplementations: recommended dose when severe deficiency | up to 5000 IU/day = 125 µg/d | up to 7000 IU/day ≈ 175 µg/d |
| Title | Design | Objective | Conclusions |
|---|---|---|---|
| The Styrian Vitamin D Hypertension Trial: effects of vitamin D on blood pressure and cardiovascular risk factors. [49] NCT02136771 | Randomized, Double-blind, Placebo-controlled, n = 200 | To assess the effects on 24-h systolic BP and diastolic BP of vitamin D3 supplementation of 2800 IU/day for 8 weeks in vitamin D deficient individuals. | There was no significant effect on systolic and diastolic BP after treatment with vitamin D supplementation. |
| Vitamin D therapy in individuals with prehypertension or hypertension: the DAYLIGHT trial. [50] NCT01240512 | Randomized, Double-blind, Parallel assignment, Multi-center, n = 534 | To compare the BP-lowering effects of high-dose (4000 IU/day) vs. low-dose (400 IU/day) of cholecalciferol for 6 months in vitamin D deficient individuals. The participants were prehypertensive or hypertensive at baseline and had not been taking antihypertensive drugs. | No significant changes in BP-measures was observed in the two groups. Nevertheless, a non-significant (p = 0.71) decrease in 24-h SBP was observed −0.8 mmHg and −1.6 mmHg in the two groups, respectively. |
| Effect of Vitamin D replacement During Winter Months in Patients With Hypertension. [51] NCT01166165 | Randomized, Double-blind, Placebo-controlled, n = 130 | To investigate therapeutic effects of 3000 IU/day cholecalciferol for 20 weeks in hypertensive patients. | In the overall group, no significant reductions in 24-h-BP, when compared to placebo. A subgroup analysis containing only deficient plasma−25(OH)D (<32 ng/mL) individuals at baseline, showed a significant reduction in 24-h systolic/diastolic BP −4/−3 mmHg. |
| The effect of vitamin D supplementation on arterial stiffness in an elderly community-based population. [52] EUDRA number: 2010–024417–31 | Randomized, Double-blind, Parallel assignment, n = 119 | To compare the effects of 50,000 IU vs. 100,000 IU single-dose intramuscular injection of cholecalciferol in a group of elderly people. | 8 weeks after treatment the group receiving high-dose of cholecalciferol had a significant improvement in arterial stiffness compared to the low-dose group. At the same time, systolic BP seemed to elevate in high-dose group. |
| Vitamin D therapy to reduce blood pressure and left ventricular hypertrophy in resistant hypertension. [53] EUDRA number: 2008-002681-63 | Randomized, Double-blind, Placebo-controlled, n = 68 | To assess effects of high-dose cholecalciferol supplementation (100,000 IU every 2nd month for 6 months) in patients treated with ≥3 antihypertensive drugs. | The study showed no improvements in systolic or diastolic BP after 6 months of treatment. |
| Cholecalciferol treatment to reduce blood pressure in older patients with isolated systolic hypertension: the VitDISH randomized controlled trial. [54] ISRCTN92186858 | Randomized, Double-blind, Placebo-controlled, n = 159 | To explore the effects on BP of high-dose cholecalciferol treatment (100,000 IU every 3rd month for 1 year) in elderly patients with isolated systolic hypertension | Treatment did not reduce BP or improve other secondary cardiovascular outcomes. |
| Vitamin D and nifedipine in the treatment of Chinese patients with grades I-II essential hypertension: a randomized placebo-controlled trial. [55] ChiCTR-ONC-13003840 | Randomized, Double-blind, Placebo-controlled, n = 126 | To assess the effects of cholecalciferol (2000 IU/day for 6 months) as ‘add on’ to nifedipine in essential hypertensive patients. | Cholecalciferol as ‘add on’ gave a significant systolic/diastolic BP reduction (−6.2/−4.2 mmHg). In subgroup analysis of vitamin D insufficient (at baseline) patients, showed −7.1/−5.7 mmHg BP reduction (p = 0.001). |
| The effect of vitamin D supplementation on blood pressure in patients with elevated blood pressure and vitamin D deficiency: a randomized, double-blind, placebo-controlled trial. [56] | Randomized, Double-blind, Placebo-controlled, n = 42 | To assess BP lowering effect of cholecalciferol 50,000 IU/week for 8 weeks in hypertensive, vitamin D deficient patients. | In the vitamin D deficient group (VDG) 92.7% of individuals recovered from insufficiency. Middle arterial pressure (MAP) decreased in average −3.7 mmHg in VDG and increased 0.9 mmHg in place-controls (p < 0.001). |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Legarth, C.; Grimm, D.; Wehland, M.; Bauer, J.; Krüger, M. The Impact of Vitamin D in the Treatment of Essential Hypertension. Int. J. Mol. Sci. 2018, 19, 455. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19020455
Legarth C, Grimm D, Wehland M, Bauer J, Krüger M. The Impact of Vitamin D in the Treatment of Essential Hypertension. International Journal of Molecular Sciences. 2018; 19(2):455. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19020455
Chicago/Turabian StyleLegarth, Christian, Daniela Grimm, Markus Wehland, Johann Bauer, and Marcus Krüger. 2018. "The Impact of Vitamin D in the Treatment of Essential Hypertension" International Journal of Molecular Sciences 19, no. 2: 455. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19020455