Structure/Function Analysis of Cotton-Based Peptide-Cellulose Conjugates: Spatiotemporal/Kinetic Assessment of Protease Aerogels Compared to Nanocrystalline and Paper Cellulose
Abstract
:1. Introduction
Nanocellulose-Based Protease Sensors
2. Results
2.1. Structure/Function and Physical Property Considerations
2.2. Structure/Function Relations of the Protease Activity at a Molecular Level
2.2.1. Cellulose Crystal Structure
2.2.2. Peptide Conformation Consideration Based on Circular Dichroism
2.2.3. Bioactivity and Kinetic Evaluation of Peptide-Cellulose Conjugates with Elastase
3. Discussion
3.1. Structural Features of Peptide-Cellulose Conjugate
3.2. Sensor Bioactivity and Kinetics
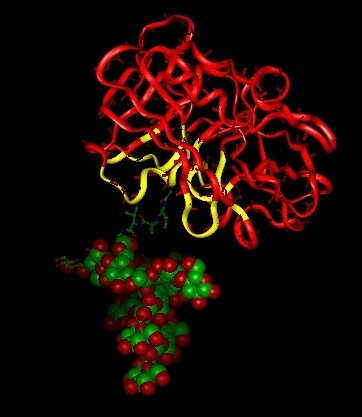
3.3. Spatiotemporal Considerations of the Peptide-Cellulose Conjugate/Elastase Interaction
4. Materials and Methods
4.1. General
4.2. Synthesis of Nanocellulosic Aerogel and Generation of Biosensors
4.3. Response and Sensitivity Assay
4.4. Surface Charge
4.5. Kinetics Assay
4.6. Circular Dichroism
4.7. X-ray Diffraction (XRD)
4.8. Rietveld Refinement Method and Crystallite Models from the NA Diffraction Pattern
4.9. Molecular Modeling Studies
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AMC | 7-amino-4-methylcoumarin |
| HNE | human neutrophil elastase |
| CFP | (cellulosic filter paper) |
| NA | nanocellulosic aerogel |
| CNC | cotton nanocrystalline cellulose |
| pCFP | peptide-cellulose conjugate on filter paper |
| pNA | peptide-cellulose conjugate on NA |
| pCNC | peptide-cellulose conjugate on NC |
| kcat | enzyme turnover rate |
| Km | enzyme binding affinity constant |
| g | gauche conformation |
| t | trans conformation |
| MAUD | materials analysis using diffraction |
References
- Dargaville, T.R.; Farrugia, B.L.; Broadbent, J.A.; Pace, S.; Upton, Z.; Voelcker, N.H. Sensors and imaging for wound healing: A review. Biosens. Bioelectron. 2013, 41, 30–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Consensus. The Role of Proteases in Wound Diagnostics; An Expert Working Group Review; Wounds International: London, UK, 2011; pp. 1–13. [Google Scholar]
- Yager, D.R.; Chen, S.M.; Ward, S.I.; Olutoye, O.O.; Diegelmann, R.F.; Kelman Cohen, I. Ability of chronic wound fluids to degrade peptide growth factors is associated with increased levels of elastase activity and diminished levels of proteinase inhibitors. Wound Repair Regen. 1997, 5, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Serena, T.E. Development of a novel technique to collect proteases from chronic wounds. Adv. Wound Care 2014, 3, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Serena, T.E.; Cullen, B.M.; Bayliff, S.W.; Gibson, M.C.; Carter, M.J.; Chen, L.; Yaakov, R.A.; Samies, J.; Sabo, M.; DeMarco, D.; et al. Defining a new diagnostic assessment parameter for wound care: Elevated protease activity, an indicator of nonhealing, for targeted protease-modulating treatment. Wound Repair Regen. 2016, 24, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, R.E.; Libert, C. Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat. Rev. Drug Discov. 2014, 13, 904–927. [Google Scholar] [CrossRef] [PubMed]
- Synder, R.J.; Driver, V.; Fife, C.E.; Lantis, J.; Peirce, B.; Serena, T.; Weir, D. Using a diagnostic tool to identify elevated protease activity levels in chronic and stalled wounds: A consensus panel discussion. Ostomy Wound Manag. 2011, 57, 36–46. [Google Scholar]
- Moore, K.; Huddleston, E.; Stacey, M.C.; Harding, K.G. Venous leg ulcers—The search for a prognostic indicator. Int. Wound J. 2007, 4, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.V.; Fontenot, K.R.; Haldane, D.; Prevost, N.T.; Condon, B.D. Human neutrophil elastase peptide sensors conjugated to cellulosic and nanocellulosic materials: Part I, synthesis and characterization of fluorescent analogs. Cellulose 2016, 23, 1283–1295. [Google Scholar] [CrossRef]
- Fontenot, K.R.; Edwards, J.V.; Haldane, D.; Graves, E.; Citron, M.S.; Prevost, N.T.; French, A.D.; Condon, B.D. Human neutrophil elastase detection with fluorescent peptide sensors conjugated to cellulosic and nanocellulosic materials: Part II, structure/function analysis. Cellulose 2016, 23, 1297–1309. [Google Scholar] [CrossRef]
- Edwards, J.V.; Prevost, N.T.; French, A.D.; Concha, M.; Condon, B.D. Kinetic and structural analysis of fluorescent peptides on cotton cellulose nanocrystals as elastase sensors. Carbohydr. Polym. 2015, 116, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.V.; Fontenot, K.R.; Prevost, N.T.; Pircher, N.; Liebner, F.; Condon, B.D. Preparation, characterization and activity of a peptide-cellulosic aerogel protease sensor from cotton. Sensors 2016, 16, 1789. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.V.; Bopp, A.; Graves, E.; Condon, B. In Vitro Hemostatic, Hydrogen Peroxide Production and Elastase Sequestration Properties of Nonwoven Ultra Clean Greige Cotton Dressing; Wound Healing Society: Orlando, FL, USA; Wound Repair and Regeneration: Orlando, FL, USA, 2013; p. A21. [Google Scholar]
- Edwards, J.V.; Fontenot, K.R.; Prevost, N.T.; Haldane, D.; Nicole, P.; Liebner, F.; French, A.D.; Condon, B.D. Protease biosensors based on peptide-nanocellulose conjugates: From molecular design to dressing interface. Int. J. Med. Nano Res. 2016. [Google Scholar] [CrossRef]
- Edwards, J.V.; Prevost, N.; French, A.D.; Concha, M.; DeLucca, A.; Wu, Q. Nanocellulose-based biosensors: Design, preparation, and activity of peptide-linked cotton cellulose nanocrystals having fluorimetric and colorimetric elastase detection sensitivity. Engineering 2013, 5, 20–28. [Google Scholar] [CrossRef]
- Reiner, R.S.; Rudie, A.W. Process scale-up of cellulose nanocrystal production to 25 kg per batch at the forest products laboratory. In Production and Applications of Cellulose Nanomaterials; Postek, M.T., Moon, R.J., Rudie, A.W., Bilodeau, M.A., Eds.; TAPPI Press Inc.: Peachtree Corners, GA, USA, 2013; pp. 21–24. [Google Scholar]
- Nam, S.; French, A.D.; Condon, B.D.; Concha, M. Segal crystallinity index revisited by the simulation of X-ray diffraction patterns of cotton cellulose Iβ and cellulose II. Carbohydr. Polym. 2016, 135, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pircher, N.; Carbajal, L.; Schimper, C.; Bacher, M.; Rennhofer, H.; Nedelec, J.-M.; Lichtenegger, H.C.; Rosenau, T.; Liebner, F. Impact of selected solvent systems on the pore and solid structure of cellulose aerogels. Cellulose 2016, 23, 1949–1966. [Google Scholar] [CrossRef] [PubMed]
- Manning, M.C.; Illangasekare, M.; Woody, R.W. Circular dichroism studies of distorted alpha-helices, twisted β-sheets, and β turns. Biophys. Chem. 1988, 31, 77–86. [Google Scholar] [CrossRef]
- Gierasch, L.M.; Deber, C.M.; Madison, V.; Niu, C.-H.; Blout, E.R. Conformations of (X-L-Pro-Y)2 cyclic hexapeptides. Preferred β-turn conformers and implications for β Turns in proteins. Biochemistry 1981, 20, 4730–4738. [Google Scholar] [CrossRef] [PubMed]
- Liebner, F.; Pircher, N.; Schimper, C.; Haimer, E.; Rosenau, T. Aerogels: Cellulose-based. In Encyclopedia of Biomedical Polymers and Polymeric Biomaterials; CRC Press: Boca Raton, FL, USA, 2015; pp. 37–75. [Google Scholar]
- Hoepfner, S.; Ratke, L.; Milow, B. Synthesis and characterisation of nanofibrillar cellulose aerogels. Cellulose 2008, 15, 121–129. [Google Scholar] [CrossRef]
- Gindl, W.; Keckes, J. All-cellulose nanocomposite. Polymer 2005, 46, 10221–10225. [Google Scholar] [CrossRef]
- Kobayashi, K.; Kimura, S.; Togawa, E.; Wada, M. Crystal transition from cellulose II hydrate to cellulose II. Carbohydr. Polym. 2011, 86, 975–981. [Google Scholar] [CrossRef]
- Liang, G.B.; Rito, C.J.; Gellman, S.H. Thermodynamic analysis of β-turn formation in Pro-Ala, Pro-Gly, and Pro-Val model peptides in methylene chloride. J. Am. Chem. Soc. 1992, 114, 4440–4442. [Google Scholar] [CrossRef]
- Edwards, J.V.; Caston-Pierre, S.; Bopp, A.F.; Goynes, W. Detection of human neutrophil elastase with peptide-bound cross-linked ethoxylate acrylate resin analogs. J. Pept. Res. 2005, 66, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Navia, M.A.; McKeever, B.M.; Springer, J.P.; Lin, T.Y.; Williams, H.R.; Fluder, E.M.; Dorn, C.P.; Hoogsteen, K. Structure of human neutrophil elastase in complex with a peptide chloromethyl ketone inhibitor at 1.84-a resolution. Proc. Natl. Acad. Sci. USA 1989, 86, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Langan, P.; Chanzy, H. Crystal structure and hydrogen-bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction. J. Am. Chem. Soc. 2002, 124, 9074–9082. [Google Scholar] [CrossRef] [PubMed]
- Yamane, C.; Aoyagi, T.; Ago, M.; Sato, K.; Okajima, K.; Takahashi, T. Two Different Surface Properties of Regenerated Cellulose due to Structural Anisotropy. Polym. J. 2006, 38, 819–826. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der Inneren Struktur und der Größe von Kolloidteilchen Mittels Röntgenstrahlen. In Kolloidchemie ein Lehrbuch; Springer: Berlin/Heidelberg, Germany, 1912; pp. 387–409. [Google Scholar]
- French, A.; Santiago Cintrón, M. Cellulose polymorphy, crystallite size, and the segal crystallinity index. Cellulose 2013, 20, 583–588. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Lutterotti, L. Total pattern fitting for the combined size-strain-stress-texture determination in thin film diffraction. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2010, 268, 334–340. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Mopac: A semiempirical molecular orbital program. J. Comput. Aided Mol. Des. 1990, 4, 1–103. [Google Scholar] [CrossRef] [PubMed]








| Name | Segal CrI (%) | Crystallite Size (Å) | Crystallite Model | Layers Molecules | Surface Charge (mV) |
|---|---|---|---|---|---|
| CFP | 87 | 69.3 | 18 | 144 | −10.2 |
| NC | 79 | 54.3 | 14 | 98 | −68 |
| NA Iβ | - | 26 | 6 | 24 | −19 |
| NA II | - | 65 | 16 | 122 | −19 |
| NA amorphous | - | 19 | 5 | 24 | −19 |
| Name | kcat (s−1) | Km (μM) | kcat/Km (M−1·s−1) | Vmax (s−1) | Corr. Coeff. (Correlation Coefficient) |
|---|---|---|---|---|---|
| Suc-APA-AMC | 2.56 | 781.4 | 3272.33 | 2.17 | 0.9515 |
| pCFP | 0.1201 | 2.150 | 5,5860.5 | 0.1021 | 0.8645 |
| pNA | 1.67 | 202 | 8267.33 | 1.42 | 0.8935 |
| pCNC | 0.7732 | 23.07 | 3,3515.39 | 0.6572 | 0.9956 |
| Name | Distance from AMC to Cellulose Floor (A) |
|---|---|
| 110 gg tripeptide | 10.7 |
| 110 gt tripeptide | 22.8 |
| 110 tg tripeptide | 18.4 |
| 1 m 10 gg tripeptide | 23.2 |
| 1 m 10 gt tripeptide | 12.2 |
| 1 m10 tg tripeptide | 9.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edwards, J.V.; Fontenot, K.; Liebner, F.; Pircher, N.D.n.; French, A.D.; Condon, B.D. Structure/Function Analysis of Cotton-Based Peptide-Cellulose Conjugates: Spatiotemporal/Kinetic Assessment of Protease Aerogels Compared to Nanocrystalline and Paper Cellulose. Int. J. Mol. Sci. 2018, 19, 840. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19030840
Edwards JV, Fontenot K, Liebner F, Pircher NDn, French AD, Condon BD. Structure/Function Analysis of Cotton-Based Peptide-Cellulose Conjugates: Spatiotemporal/Kinetic Assessment of Protease Aerogels Compared to Nanocrystalline and Paper Cellulose. International Journal of Molecular Sciences. 2018; 19(3):840. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19030840
Chicago/Turabian StyleEdwards, J. Vincent, Krystal Fontenot, Falk Liebner, Nicole Doyle nee Pircher, Alfred D. French, and Brian D. Condon. 2018. "Structure/Function Analysis of Cotton-Based Peptide-Cellulose Conjugates: Spatiotemporal/Kinetic Assessment of Protease Aerogels Compared to Nanocrystalline and Paper Cellulose" International Journal of Molecular Sciences 19, no. 3: 840. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19030840