The Amino-Terminal Domain of GRK5 Inhibits Cardiac Hypertrophy through the Regulation of Calcium-Calmodulin Dependent Transcription Factors
Abstract
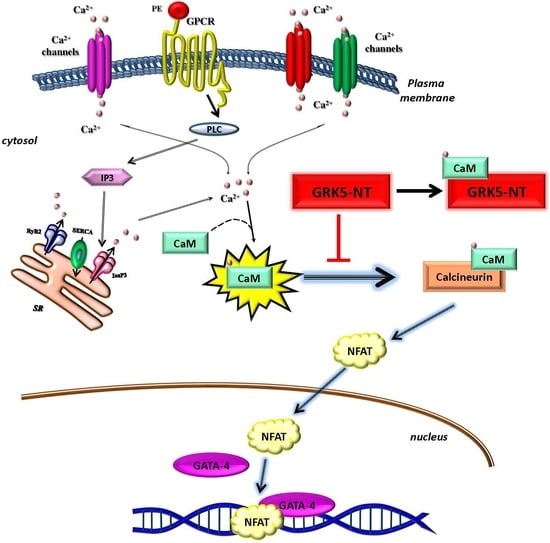
:1. Introduction
2. Results
2.1. GRK5-NT Regulates the Activation of Calcium-Calmodulin Dependent Transcription Factors In Vitro
2.2. GRK5-NT Inhibits NFAT Activation by Competing for Binding to Calmodulin
2.3. GRK5-NT Regulates the Activation of Calcium-Calmodulin Dependent Transcription Factors In Vivo
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Plasmids
4.3. Western Blot
4.4. In Vivo Study
4.5. Cardiac Ultrasounds (CUS)
4.6. EMSA
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Author contributions
Conflicts of interest
References
- Devereux, R.B.; Roman, M.J. Left ventricular hypertrophy in hypertension: Stimuli, patterns, and consequences. Hypertens. Res. 1999, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Schmieder, R.E.; Messerli, F.H. Hypertension and the heart. J. Hum. Hypertens. 2000, 14, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Santulli, G.; Iaccarino, G. Adrenergic signaling in heart failure and cardiovascular aging. Maturitas 2016, 93, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Vakili, B.A.; Okin, P.M.; Devereux, R.B. Prognostic implications of left ventricular hypertrophy. Am. Heart J. 2001, 141, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Hardt, S.E.; Sadoshima, J. Negative regulators of cardiac hypertrophy. Cardiovasc. Res. 2004, 63, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Sadoshima, J.; Izumo, S. The cellular and molecular response of cardiac myocytes to mechanical stress. Annu. Rev. Physiol. 1997, 59, 551–571. [Google Scholar] [CrossRef] [PubMed]
- Molkentin, J.D.; Dorn, G.W., II. Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu. Rev. Physiol. 2001, 63, 391–426. [Google Scholar] [CrossRef] [PubMed]
- Heineke, J.; Molkentin, J.D. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat. Rev. Mol. Cell Biol. 2006, 7, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, J.; Trimarco, B.; Iaccarino, G.; Santulli, G. New Insights in Cardiac Calcium Handling and Excitation-Contraction Coupling. Adv. Exp. Med. Biol. 2017. [Google Scholar] [CrossRef]
- Santulli, G.; Nakashima, R.; Yuan, Q.; Marks, A.R. Intracellular calcium release channels: An update. J. Physiol. 2017, 595, 3041–3051. [Google Scholar] [CrossRef] [PubMed]
- Akazawa, H.; Komuro, I. Roles of cardiac transcription factors in cardiac hypertrophy. Circ. Res. 2003, 92, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R.A.; Yutzey, K.E. Calcineurin signaling and NFAT activation in cardiovascular and skeletal muscle development. Dev. Biol. 2004, 266, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Yang, J.; Santulli, G.; Reiken, S.R.; Wronska, A.; Kim, M.M.; Osborne, B.W.; Lacampagne, A.; Yin, Y.; Marks, A.R. Maintenance of normal blood pressure is dependent on IP3R1-mediated regulation of eNOS. Proc. Nat. Acad. Sci. USA 2016, 113, 8532–8537. [Google Scholar] [CrossRef] [PubMed]
- Hogan, P.G.; Chen, L.; Nardone, J.; Rao, A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003, 17, 2205–2232. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Wiese, R.J.; Bueno, O.F.; Dai, Y.S.; Markham, B.E.; Molkentin, J.D. The transcription factor GATA4 is activated by extracellular signal-regulated kinase 1- and 2-mediated phosphorylation of serine 105 in cardiomyocytes. Mol. Cell Biol. 2001, 21, 7460–7469. [Google Scholar] [CrossRef] [PubMed]
- Sorriento, D.; Ciccarelli, M.; Santulli, G.; Campanile, A.; Altobelli, G.G.; Cimini, V.; Galasso, G.; Astone, D.; Piscione, F.; Pastore, L.; et al. The G-protein-coupled receptor kinase 5 inhibits NFκB transcriptional activity by inducing nuclear accumulation of IκBα. Proc. Nat. Acad. Sci. USA 2008, 105, 17818–17823. [Google Scholar] [CrossRef] [PubMed]
- Sorriento, D.; Illario, M.; Finelli, R.; Iaccarino, G. To NFκB or not to NFκB: The Dilemma on How to Inhibit a Cancer Cell Fate Regulator. Transl. Med. UniSa 2012, 4, 73–85. [Google Scholar] [PubMed]
- Sorriento, D.; Campanile, A.; Santulli, G.; Leggiero, E.; Pastore, L.; Trimarco, B.; Iaccarino, G. A new synthetic protein, TAT-RH, inhibits tumor growth through the regulation of NFκB activity. Mol. Cancer 2009, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Sorriento, D.; Santulli, G.; Fusco, A.; Anastasio, A.; Trimarco, B.; Iaccarino, G. Intracardiac injection of AdGRK5-NT reduces left ventricular hypertrophy by inhibiting NFκB-dependent hypertrophic gene expression. Hypertension 2010, 56, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Pronin, A.N.; Satpaev, D.K.; Slepak, V.Z.; Benovic, J.L. Regulation of G protein-coupled receptor kinases by calmodulin and localization of the calmodulin binding domain. J. Biol. Chem. 1997, 272, 18273–18280. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.R.; Scott, M.G.; Pitcher, J.A. G protein-coupled receptor kinase 5 contains a DNA-binding nuclear localization sequence. Mol. Cell Biol. 2004, 24, 10169–10179. [Google Scholar] [CrossRef] [PubMed]
- Frey, N.; Olson, E.N. Cardiac hypertrophy: The good, the bad, and the ugly. Annu. Rev. Physiol. 2003, 65, 45–79. [Google Scholar] [CrossRef] [PubMed]
- Russell, B.; Motlagh, D.; Ashley, W.W. Form follows function: How muscle shape is regulated by work. J. Appl. Physiol. 2000, 88, 1127–1132. [Google Scholar] [CrossRef] [PubMed]
- Molkentin, J.D.; Lu, J.R.; Antos, C.L.; Markham, B.; Richardson, J.; Robbins, J.; Grant, S.R.; Olson, E.N. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 1998, 93, 215–228. [Google Scholar] [CrossRef]
- Gordon, J.W.; Shaw, J.A.; Kirshenbaum, L.A. Multiple facets of NF-κB in the heart: To be or not to NF-κB. Circ. Res. 2011, 108, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, Y.; Auger-Messier, M.; Molkentin, J.D. Interaction between NFκB and NFAT coordinates cardiac hypertrophy and pathological remodeling. Circ. Res. 2012, 110, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Martini, J.S.; Raake, P.; Vinge, L.E.; DeGeorge, B.R., Jr.; Chuprun, J.K.; Harris, D.M.; Gao, E.; Eckhart, A.D.; Pitcher, J.A.; Koch, W.J. Uncovering G protein-coupled receptor kinase-5 as a histone deacetylase kinase in the nucleus of cardiomyocytes. Proc. Nat. Acad. Sci USA 2008, 105, 12457–12462. [Google Scholar] [CrossRef] [PubMed]
- Belmonte, S.L.; Blaxall, B.C. G protein-coupled receptor kinase 5: Exploring its hype in cardiac hypertrophy. Circ. Res. 2012, 111, 957–958. [Google Scholar] [CrossRef] [PubMed]
- Hullmann, J.E.; Grisanti, L.A.; Makarewich, C.A.; Gao, E.; Gold, J.I.; Chuprun, J.K.; Tilley, D.G.; Houser, S.R.; Koch, W.J. GRK5-mediated exacerbation of pathological cardiac hypertrophy involves facilitation of nuclear NFAT activity. Circ. Res. 2014, 115, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Dzimiri, N.; Muiya, P.; Andres, E.; Al-Halees, Z. Differential functional expression of human myocardial G protein receptor kinases in left ventricular cardiac diseases. Eur. J. Pharmacol. 2004, 489, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Sorriento, D.; Santulli, G.; Del Giudice, C.; Anastasio, A.; Trimarco, B.; Iaccarino, G. Endothelial cells are able to synthesize and release catecholamines both in vitro and in vivo. Hypertension 2012, 60, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Santulli, G.; Campanile, A.; Spinelli, L.; Assante di Panzillo, E.; Ciccarelli, M.; Trimarco, B.; Iaccarino, G. G protein-coupled receptor kinase 2 in patients with acute myocardial infarction. Am. J. Cardiol. 2011, 107, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Ciccarelli, M.; Sorriento, D.; Cipolletta, E.; Santulli, G.; Fusco, A.; Zhou, R.H.; Eckhart, A.D.; Peppel, K.; Koch, W.J.; Trimarco, B.; et al. Impaired neoangiogenesis in β2-adrenoceptor gene-deficient mice: Restoration by intravascular human β2-adrenoceptor gene transfer and role of NFκB and CREB transcription factors. Br. J. Pharmacol. 2011, 162, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Santulli, G.; Basilicata, M.F.; De Simone, M.; Del Giudice, C.; Anastasio, A.; Sorriento, D.; Saviano, M.; Del Gatto, A.; Trimarco, B.; Pedone, C.; et al. Evaluation of the anti-angiogenic properties of the new selective αVβ3 integrin antagonist RGDechiHCit. J. Transl. Med. 2011, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Iaccarino, G.; Izzo, R.; Trimarco, V.; Cipolletta, E.; Lanni, F.; Sorriento, D.; Iovino, G.L.; Rozza, F.; De Luca, N.; Priante, O.; et al. β2-adrenergic receptor polymorphisms and treatment-induced regression of left ventricular hypertrophy in hypertension. Clin. Pharmacol. Ther. 2006, 80, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Santulli, G.; Cipolletta, E.; Sorriento, D.; Del Giudice, C.; Anastasio, A.; Monaco, S.; Maione, A.S.; Condorelli, G.; Puca, A.; Trimarco, B.; et al. CaMK4 Gene Deletion Induces Hypertension. J. Am. Heart Assoc. 2012, 1, e001081. [Google Scholar] [CrossRef] [PubMed]
- Santulli, G.; Xie, W.; Reiken, S.R.; Marks, A.R. Mitochondrial calcium overload is a key determinant in heart failure. Proc. Nat. Acad. Sci. USA 2015, 112, 11389–11394. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorriento, D.; Santulli, G.; Ciccarelli, M.; Maione, A.S.; Illario, M.; Trimarco, B.; Iaccarino, G. The Amino-Terminal Domain of GRK5 Inhibits Cardiac Hypertrophy through the Regulation of Calcium-Calmodulin Dependent Transcription Factors. Int. J. Mol. Sci. 2018, 19, 861. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19030861
Sorriento D, Santulli G, Ciccarelli M, Maione AS, Illario M, Trimarco B, Iaccarino G. The Amino-Terminal Domain of GRK5 Inhibits Cardiac Hypertrophy through the Regulation of Calcium-Calmodulin Dependent Transcription Factors. International Journal of Molecular Sciences. 2018; 19(3):861. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19030861
Chicago/Turabian StyleSorriento, Daniela, Gaetano Santulli, Michele Ciccarelli, Angela Serena Maione, Maddalena Illario, Bruno Trimarco, and Guido Iaccarino. 2018. "The Amino-Terminal Domain of GRK5 Inhibits Cardiac Hypertrophy through the Regulation of Calcium-Calmodulin Dependent Transcription Factors" International Journal of Molecular Sciences 19, no. 3: 861. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19030861