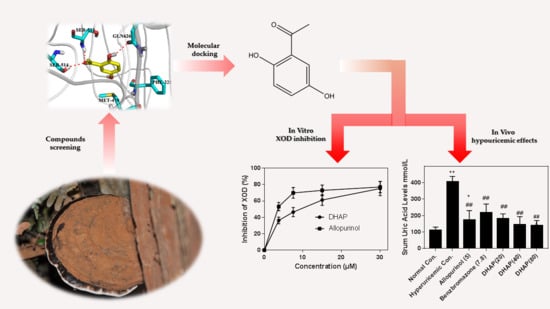
Hypouricemic Effect of 2,5-Dihydroxyacetophenone, a Computational Screened Bioactive Compound from Ganoderma applanatum, on Hyperuricemic Mice
Abstract
:1. Introduction
2. Results
2.1. In Vitro Enzyme Activity Assay
2.2. Animal Experiment
2.3. Computational Studies
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. In Vitro XOD Inhibition Assay
4.3. Animals
4.4. Drug Administration
4.5. Mensuration of Uric Acid Level, XOD Activities, BUN, and Creatinine Levels
4.6. Organ Coefficients
4.7. Statistical Analysis
4.8. RT-PCR Analysis of URAT1, GLUT9, OAT1, and CNT2
4.9. Western Blot Analysis
4.10. Molecular Docking
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MSU | monosodium urate |
| SUA | serum uric acid |
| HUA | hyperuricemia |
| XOD | xanthine oxidase |
| GLUT9 | glucose transporter 9 |
| OAT1 | organic anion transporter 1 |
| DHAP | 2,5-dihydroxyacetophenone |
| BUN | blood urea nitrogen |
| URAT1 | uric acid transporter 1 |
| CNT2 | gastrointestinal concentrative nucleoside transporter 2 |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| PO | potassium oxonate |
| HX | hypoxanthine |
References
- Dalbeth, N.; Merriman, T.R.; Stamp, L.K. Gout. Lancet 2016, 388, 2039–2052. [Google Scholar] [CrossRef]
- Rock, K.L.; Kataoka, H.; Lai, J.J. Uric acid as a danger signal in gout and its comorbidities. Nat. Rev. Rheumatol. 2013, 9, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Abhishek, A.; Roddy, E.; Doherty, M. Gout—A guide for the general and acute physicians. Clin. Med. 2017, 17, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, X.M.; Wang, Y.L.; Liu, B.C. Prevalence of hyperuricemia among Chinese adults: A national cross-sectional survey using multistage, stratified sampling. J. Nephrol. 2014, 27, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Kuwata, H.; Okamura, S.; Hayashino, Y.; Tsujii, S.; Ishii, H. Serum uric acid levels are associated with increased risk of newly developed diabetic retinopathy among Japanese male patients with type 2 diabetes: A prospective cohort study (diabetes distress and care registry at Tenri [DDCRT 13]). Diabetes Metab. Res. 2017, 33. [Google Scholar] [CrossRef] [PubMed]
- Barkas, F.; Elisaf, M. National hyperlipidemia management policies improve lipid target attainment in clinical practice. Curr. Med. Res. Opin. 2018, 34, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Hu, J.; Song, N.; Chen, R.; Zhang, T.; Ding, X. Hyperuricemia increases the risk of acute kidney injury: A systematic review and meta-analysis. BMC Nephrol. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Shahid, H.; Singh, J.A. Investigational drugs for hyperuricemia. Expert Opin. Investig. Drugs 2015, 24, 1013–1030. [Google Scholar] [CrossRef] [PubMed]
- Crittenden, D.B.; Pillinger, M.H. New therapies for gout. Annu. Rev. Med. 2013, 64, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, D.; Yu, B.; Qi, J. Screening inhibitors of xanthine oxidase from natural products using enzyme immobilized magnetic beads by high-performance liquid chromatography coupled with tandem mass spectrometry. J. Sep. Sci. 2017, 40, 1877–1886. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, C.; Wang, S.; Mi, C.; He, Y.; Zhang, J.; Zhang, Y.; Anderson, S.; Yuan, C. Anti-hyperuricemia effects of allopurinol are improved by Smilax riparia, a traditional Chinese herbal medicine. J. Ethnopharmacol. 2015, 162, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Fugal Resource; China Agriculture Press: Beijing, China, 2013. [Google Scholar]
- Paterson, R.R. Ganoderma—A therapeutic fungal biofactory. Phytochemistry 2006, 67, 1985–2001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baby, S.; Johnson, A.J.; Govindan, B. Secondary metabolites from Ganoderma. Phytochemistry 2015, 114, 66–101. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhao, C.; Pan, W.; Wang, J.; Wang, W. Carboxylate groups play a major role in antitumor activity of Ganoderma applanatum polysaccharide. Carbohydr. Polym. 2015, 123, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Liu, C.; Qin, Z.; Jiang, J.; Sun, Y. Ganoderma applanatum terpenes protect mouse liver against benzo(α)pyren-induced oxidative stress and inflammation. Environ. Toxicol. Pharmacol. 2011, 31, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Elkhateeb, W.A.; Zaghlol, G.M.; El-Garawani, I.M.; Ahmed, E.F.; Rateb, M.E.; Moneim, A.E.A. Ganoderma applanatum secondary metabolites induced apoptosis through different pathways: In vivo and in vitro anticancer studies. Biomed. Pharmacother. 2018, 101, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Manayi, A.; Vazirian, M.; Zade, F.H.; Tehranifard, A. Immunomodulation effect of aqueous extract of the Artist’s conk medicinal mushroom, Ganoderma applanatum (agaricomycetes), on the rainbow trout (Oncorhynchus mykiss). Int. J. Med. Mushrooms 2016, 18, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Yong, T.; Chen, S.; Xie, Y.; Chen, D.; Su, J.; Shuai, O.; Jiao, C.; Zuo, D. Hypouricemic effects of Ganoderma applanatum in hyperuricemia mice through OAT1 and GLUT9. Front. Pharmacol. 2017, 8, 996. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Shim, S.H.; Kim, J.S.; Shin, K.H.; Kang, S.S. Aldose reductase inhibitors from the fruiting bodies of Ganoderma applanatum. Biol. Pharm. Bull. 2005, 28, 1103–1105. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.F.; Rudan, I.; Hastie, N.D.; Campbell, H. A ‘complexity’ of urate transporters. Kidney Int. 2010, 78, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Tatani, K.; Hiratochi, M.; Nonaka, Y.; Isaji, M.; Shuto, S. Identification of 8-aminoadenosine derivatives as a new class of human concentrative nucleoside transporter 2 inhibitors. ACS Med. Chem. Lett. 2015, 6, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Lee, J.H.; Jung, S.H.; Lee, S.; Chinnathambi, A.; Alharbi, S.A.; Yang, W.M.; Um, J.; Sethi, G.; Ahn, K.S. 2,5-dihydroxyacetophenone induces apoptosis of multiple myeloma cells by regulating the MAPK activation pathway. Molecules 2017, 22, 1157. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Jung, H.W.; Lee, J.Y.; Kim, J.S.; Kang, S.S.; Kim, Y.S.; Park, Y. 2,5-dihydroxyacetophenone isolated from Rehmanniae Radix Preparata inhibits inflammatory responses in lipopolysaccharide-stimulated RAW264.7 macrophages. J. Med. Food 2012, 15, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, R.; Ronco, C.; Kellum, J.A.; Mehta, R.L.; Palevsky, P. Acute renal failure–definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit. Care 2004, 8, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/10279 (accessed on 25 April 2018).
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2094 (accessed on 25 April 2018).
- Pineda, C.; Fuentes-Gómez, A.J.; Hernández-Díaz, C.; Zamudio-Cuevas, Y.; Fernández-Torres, J.; López-Macay, A.; Alba-Sánchez, I.; Camacho-Galindo, J.; Ventura, L.; Gómez-Quiróz, L.E.; et al. Animal model of acute gout reproduces the inflammatory and ultrasonographic joint changes of human gout. Arthritis Res. Ther. 2015, 17, 37. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Matsumura, T.; Ichida, K.; Okamoto, K.; Nishino, T. Human xanthine oxidase changes its substrate specificity to aldehyde oxidase type upon mutation of amino acid residues in the active site: Roles of active site residues in binding and activation of purine substrate. J. Biochem. 2007, 141, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Nepali, K.; Agarwal, A.; Sapra, S.; Mittal, V.; Kumar, R.; Banerjee, U.C.; Gupta, M.K.; Satti, N.K.; Suri, O.P.; Dhar, K.L. N-(1,3-Diaryl-3-oxopropyl) amides as a new template for xanthine oxidase inhibitors. Bioorg. Med. Chem. 2011, 19, 5569–5576. [Google Scholar] [CrossRef] [PubMed]
- Ichida, K.; Matsuo, H.; Takada, T.; Nakayama, A.; Murakami, K.; Shimizu, T.; Yamanashi, Y.; Kasuga, H.; Nakashima, H.; Nakamura, T. Decreased extra-renal urate excretion is a common cause of hyperuricemia. Nat. Commun. 2012, 3, 764. [Google Scholar] [CrossRef] [PubMed]
- Otani, N.; Ouchi, M.; Hayashi, K.; Jutabha, P.; Anzai, N. Roles of organic anion transporters (OATs) in renal proximal tubules and their localization. Anat. Sci. Int. 2017, 92, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Vitart, V.; Rudan, I.; Hayward, C.; Gray, N.K.; Floyd, J.; Palmer, C.N.; Knott, S.A.; Kolcic, I.; Polasek, O.; Graessler, J. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat. Genet. 2008, 40, 437. [Google Scholar] [CrossRef] [PubMed]
- So, A.; Thorens, B. Uric acid transport and disease. J. Clin. Investig. 2010, 120, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.; Awale, S.; Tezuka, Y.; Tran, Q.L.; Watanabe, H.; Kadota, S. Xanthine oxidase inhibitory activity of Vietnamese medicinal plants. Biol. Pharm. Bull. 2004, 27, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Yong, T.; Zhang, M.; Chen, D.; Shuai, O.; Chen, S.; Su, J.; Jiao, C.; Feng, D.; Xie, Y. Actions of water extract from Cordyceps militaris in hyperuricemic mice induced by potassium oxonate combined with hypoxanthine. J. Ethnopharmacol. 2016, 194, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Protein Data Bank. Available online: https://www.rcsb.org/structure/1FIQ (accessed on 25 April 2018).
- Li, H.; Zhao, M.; Su, G.; Lin, L.; Wang, Y. Effect of Soy Sauce on Serum Uric Acid Levels in Hyperuricemic Rats and Identification of Flazin as a Potent Xanthine Oxidase Inhibitor. J. Agric. Food Chem. 2016, 64, 4725–4734. [Google Scholar] [CrossRef] [PubMed]









| Ligands | Docking Score | XP Gscore | Glide Emodel |
|---|---|---|---|
| DHAP | −5.366 | −5.426 | −35.140 |
| Allopurinol | −6.170 | −6.214 | −42.933 |
| Description | GenBank | Primer Name | Primer Sequences (5′-3′) | Product Size (bp) | Tm (°C) | Thermal Cycles |
|---|---|---|---|---|---|---|
| GAPDH a | NM_008084.2 | M-GAPDH-S | AGGAGCGAGACCCCACTAACA | 247 | 60 | 40 |
| M-GAPDH-A | AGGGGGGCTAAGCAGTTGGT | 60 | 40 | |||
| GLUT9 b | NM_001012363.2 | M-SLC2A9-S | GATGCTCATTGTGGGACGGTT | 241 | 60 | 40 |
| M-SLC2A9-A | CTGGACCAAGGCAGGGACAA | 60 | 40 | |||
| URAT1 c | NM_009203.3 | M-SLC22A12-S | CGCTTCCGACAACCTCAATG | 254 | 60 | 40 |
| M-SLC22A12-A | CTTCTGCGCCCAAACCTATCT | 60 | 40 | |||
| OAT1 d | NM_008766.3 | M-SLC22A6-S | GCCTTGATGGCTGGGTCTATG | 287 | 60 | 40 |
| M-SLC22A6-A | AGCCAAAGACATGCCCGAGA | 60 | 40 | |||
| CNT2 e | NM_172980.2 | M-CNT2-S | TTGGGCAAAGCGGGTGTT | 135 | 60 | 40 |
| M-CNT2-A | CAGGCAAAGAGGATGAGGATGA | 60 | 40 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, D.; Yong, T.; Chen, S.; Xie, Y.; Chen, D.; Zhou, X.; Li, D.; Li, M.; Su, L.; Zuo, D. Hypouricemic Effect of 2,5-Dihydroxyacetophenone, a Computational Screened Bioactive Compound from Ganoderma applanatum, on Hyperuricemic Mice. Int. J. Mol. Sci. 2018, 19, 1394. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19051394
Liang D, Yong T, Chen S, Xie Y, Chen D, Zhou X, Li D, Li M, Su L, Zuo D. Hypouricemic Effect of 2,5-Dihydroxyacetophenone, a Computational Screened Bioactive Compound from Ganoderma applanatum, on Hyperuricemic Mice. International Journal of Molecular Sciences. 2018; 19(5):1394. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19051394
Chicago/Turabian StyleLiang, Danling, Tianqiao Yong, Shaodan Chen, Yizhen Xie, Diling Chen, Xinxin Zhou, Dan Li, Muxia Li, Lu Su, and Dan Zuo. 2018. "Hypouricemic Effect of 2,5-Dihydroxyacetophenone, a Computational Screened Bioactive Compound from Ganoderma applanatum, on Hyperuricemic Mice" International Journal of Molecular Sciences 19, no. 5: 1394. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19051394