A Synthetic Peptide AWRK6 Alleviates Lipopolysaccharide-Induced Liver Injury
Abstract
:1. Introduction
2. Results
2.1. AWRK6 Relieved LPS-Induced Liver Injury in Mice
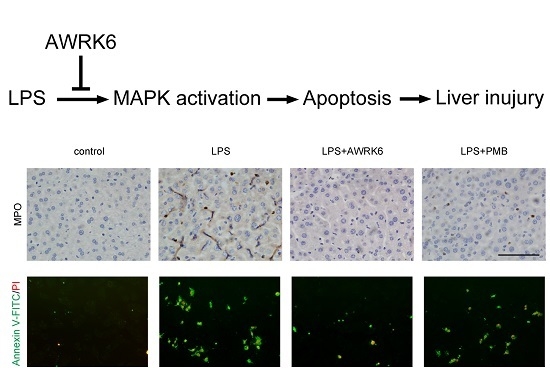
2.2. AWRK6 Inhibited LPS-Induced Liver Cell Apoptosis in Mice
2.3. AWRK6 Inhibited LPS-Induced Liver Cell Apoptosis in HepG2 Cells
2.4. MAPKs Were Involved in the Protection of AWRK6 against Liver Injury
3. Discussion
4. Materials and Methods
4.1. Mice Model
4.2. ALT, SOD and iNOS Assay
4.3. Histopathological Examination
4.4. TUNEL Assay
4.5. Western Blotting
4.6. Cell Culture
4.7. Cell Viability Assay
4.8. Annexin V-FITC/PI Staining
4.9. Reactive Oxygen Species Assay
4.10. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Wang, W.; Fang, H.; Yang, Y.; Jiang, X.; Liu, S.; Hu, J.; Hu, Q.; Dahmen, U.; Dirsch, O. Baicalein protects against polymicrobial sepsis-induced liver injury via inhibition of inflammation and apoptosis in mice. Eur. J. Pharmacol. 2015, 748, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Arumanayagam, S.; Arunmani, M. Hepatoprotective and antibacterial activity of lippia nodiflora linn. Against lipopolysaccharides on HepG2 cells. Pharmacogn. Mag. 2015, 11, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Baranova, I.N.; Souza, A.C.P.; Bocharov, A.V.; Vishnyakova, T.G.; Hu, X.; Vaisman, B.L.; Amar, M.J.; Chen, Z.; Kost, Y.; Remaley, A.T.; et al. Human sr-bi and sr-bii potentiate lipopolysaccharide-induced inflammation and acute liver and kidney injury in mice. J. Immunol. 2016, 196, 3135–3147. [Google Scholar] [CrossRef] [PubMed]
- Vardakas, K.Z.; Falagas, M.E. Colistin versus polymyxin b for the treatment of patients with multidrug-resistant gram-negative infections: A systematic review and meta-analysis. Int. J. Antimicrob. Agents 2017, 49, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Nation, R.L.; Li, J.; Cars, O.; Couet, W.; Dudley, M.N.; Kaye, K.S.; Mouton, J.W.; Paterson, D.L.; Tam, V.H.; Theuretzbacher, U.; et al. Framework for optimisation of the clinical use of colistin and polymyxin b: The prato polymyxin consensus. Lancet Infect. Dis. 2015, 15, 225–234. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Sherwood, E.R. Getting sepsis therapy right. Science 2015, 347, 1201–1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haney, E.F.; Mansour, S.C.; Hancock, R.E.W. Antimicrobial Peptides: An introduction. In Antimicrobial Peptides: Methods and Protocols; Hansen, P.R., Ed.; Springer: New York, NY, USA, 2017; pp. 3–22. [Google Scholar]
- Hu, Z.; Murakami, T.; Suzuki, K.; Tamura, H.; Reich, J.; Kuwahara-Arai, K.; Iba, T.; Nagaoka, I. Antimicrobial cathelicidin peptide ll-37 inhibits the pyroptosis of macrophages and improves the survival of polybacterial septic mice. Int. Immunol. 2016, 28, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shang, D. Inhibitory effects of antimicrobial peptides on lipopolysaccharide-induced inflammation. Mediators Inflamm. 2015, 2015, 167572. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Li, Q.; Song, S.; Feng, K.; Zhang, D.; Wang, Q.; Chen, Y. Characterization of antimicrobial peptides isolated from the skin of the chinese frog, rana dybowskii. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2009, 154, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Wang, Z.; Jin, L.; Li, H.; Hwang, J.; Hanrahan, J.W.; Wang, Q. Development of a novel antimicrobial peptide awrk6. Bang. J. Pharmacol. 2016, 11, 460. [Google Scholar] [CrossRef]
- Wang, Q.; Jin, L.; Wang, H.; Tai, S.; Liu, H.; Zhang, D. Awrk6, a synthetic cationic peptide derived from antimicrobial peptide dybowskin-2cdya, inhibits lipopolysaccharide-induced inflammatory response. Int. J. Mol. Sci. 2018, 19, 600. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.M.; Srivastava, S.; Singh, M.; Bajpai, V.K.; Ghosh, J.K. Consequences of alteration in leucine zipper sequence of melittin in its neutralization of lipopolysaccharide-induced proinflammatory response in macrophage cells and interaction with lipopolysaccharide. J. Biol. Chem. 2012, 287, 1980–1995. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.W.; Scott, M.G. The role of antimicrobial peptides in animal defenses. Proc. Natl. Acad. Sci. USA 2000, 97, 8856–88616. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, G.; Zhao, E.; Liu, K.; Lin, Y.; Tesfa, L.; Tanaka, K.E.; Czaja, M.J. Macrophage autophagy limits acute toxic liver injury in mice through down regulation of interleukin-1β. J. Hepatol. 2016, 64, 118–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwakiri, Y.; Kim, M.Y. Nitric oxide in liver diseases. Trends Pharmacol. Sci. 2015, 36, 524–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Z.; Gong, X.; Yang, Y.; Huang, L.; Zhang, Q.; Zhang, P.; Wan, R.; Zhang, B. Hepatoprotective effect of quercetin against lps/d-galn induced acute liver injury in mice by inhibiting the ikk/nf-κb and mapk signal pathways. Int. Immunopharmacol. 2017, 52, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Qian, K.; Xiong, J.; Ma, K.; Wang, A.; Zou, Y. Curcumin alleviates lipopolysaccharide induced sepsis and liver failure by suppression of oxidative stress-related inflammation via pi3k/akt and nf-κb related signaling. Biomed. Pharmacother. 2016, 83, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wohland, T.; Ho, B.; Ding, J.L. Perturbation of lipopolysaccharide (lps) micelles by sushi 3 (s3) antimicrobial peptide. The importance of an intermolecular disulfide bond in s3 dimer for binding, disruption and neutralization of lps. J. Biol. Chem. 2004, 279, 50150–50156. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K.; Sugishita, K.; Miyajima, K. Interactions of an antimicrobial peptide, magainin 2, with lipopolysaccharide-containing liposomes as a model for outer membranes of gram-negative bacteria. FEBS Lett. 1999, 449, 221–224. [Google Scholar] [CrossRef]
- Levin, M.; Quint, P.A.; Goldstein, B.; Barton, P.; Bradley, J.S.; Shemie, S.D.; Yeh, T.; Kim, S.S.; Cafaro, D.P.; Scannon, P.J.; et al. Recombinant bactericidal/permeability-increasing protein (rbpi21) as adjunctive treatment for children with severe meningococcal sepsis: A randomised trial. Rbpi21 meningococcal sepsis study group. Lancet 2000, 356, 961–967. [Google Scholar] [CrossRef]
- Martin, L.; van Meegern, A.; Doemming, S.; Schuerholz, T. Antimicrobial peptides in human sepsis. Front. Immunol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Ajji, P.K.; Binder, M.J.; Walder, K.; Puri, M. Balsamin induces apoptosis in breast cancer cells via DNA fragmentation and cell cycle arrest. Mol. Cell. Biochem. 2017, 432, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Kramer, D.; Stark, N.; Schulz-Heddergott, R.; Erytch, N.; Edmunds, S.; Roßmann, L.; Bastians, H.; Concin, N.; Moll, U.M.; Dobbelstein, M. Strong antitumor synergy between DNA crosslinking and hsp90 inhibition causes massive premitotic DNA fragmentation in ovarian cancer cells. Cell Death Differ. 2016, 24, 300. [Google Scholar] [CrossRef] [PubMed]
- Lemke, G. Phosphatidylserine is the signal for tam receptors and their ligands. Trends Biochem. Sci. 2017, 42, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Masaki, T.; Chiba, S.; Tatsukawa, H.; Yasuda, T.; Noguchi, H.; Seike, M.; Yoshimatsu, H. Adiponectin protects lps-induced liver injury through modulation of tnf-α in kk-ay obese mice. Hepatology 2004, 40, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of apoptosis by the bcl-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2013, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Hata, A.N.; Engelman, J.A.; Faber, A.C. The bcl2 family: Key mediators of the apoptotic response to targeted anticancer therapeutics. Cancer Discov. 2015, 5, 475. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, W.A.; Ahad, A.; Ahsan, H. The mystery of bcl2 family: Bcl-2 proteins and apoptosis: An update. Arch. Toxicol. 2015, 89, 289–317. [Google Scholar] [CrossRef] [PubMed]
- Renault, T.T.; Floros, K.V.; Elkholi, R.; Corrigan, K.-A.; Kushnareva, Y.; Wieder, S.Y.; Lindtner, C.; Serasinghe, M.N.; Asciolla, J.J.; Buettner, C.; et al. Mitochondrial shape governs bax-induced membrane permeabilization and apoptosis. Mol. Cell 2015, 57, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Choy, K.W.; Lau, Y.S.; Murugan, D.; Vanhoutte, P.M.; Mustafa, M.R. Paeonol attenuates lps-induced endothelial dysfunction and apoptosis by inhibiting bmp4 and tlr4 signaling simultaneously but independently. J. Pharmacol. Exp. Ther. 2018, 364, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, W.-Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.-F. Signaling pathway of mapk/erk in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. 2015, 35, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Fortier, M.; Cadoux, M.; Boussetta, N.; Couty, J.P.; Desdouets, C.; Celton-Morizur, S. P38-alpha mapk couples inflammatory response and proliferation of hepatocytes during acute liver injury. J. Hepatol. 2017, 66, S645. [Google Scholar] [CrossRef]
- Dewanjee, S.; Joardar, S.; Bhattacharjee, N.; Dua, T.K.; Das, S.; Kalita, J.; Manna, P. Edible leaf extract of ipomoea aquatica forssk. (convolvulaceae) attenuates doxorubicin-induced liver injury via inhibiting oxidative impairment, mapk activation and intrinsic pathway of apoptosis. Food Chem. Toxicol. 2017, 105, 322–336. [Google Scholar] [CrossRef] [PubMed]
- Low, H.B.; Zhang, Y. Regulatory roles of mapk phosphatases in cancer. Immune Netw. 2016, 16, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Ishida, W.; Harada, Y.; Fukuda, K.; Fukushima, A. Inhibition by the antimicrobial peptide ll37 of lipopolysaccharide-induced innate immune responses in human corneal fibroblasts. Invest. Ophthalmol. Vis. Sci. 2016, 57, 30–39. [Google Scholar] [PubMed]
- Park, H.J.; Lee, H.J.; Choi, M.S.; Son, D.J.; Song, H.S.; Song, M.J.; Lee, J.M.; Han, S.B.; Kim, Y.; Hong, J.T. Jnk pathway is involved in the inhibition of inflammatory target gene expression and nf-kappab activation by melittin. J. Inflamm. (Lond.) 2008, 5, 7. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, L.; Wang, Q.; Zhang, H.; Tai, S.; Liu, H.; Zhang, D. A Synthetic Peptide AWRK6 Alleviates Lipopolysaccharide-Induced Liver Injury. Int. J. Mol. Sci. 2018, 19, 2661. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19092661
Jin L, Wang Q, Zhang H, Tai S, Liu H, Zhang D. A Synthetic Peptide AWRK6 Alleviates Lipopolysaccharide-Induced Liver Injury. International Journal of Molecular Sciences. 2018; 19(9):2661. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19092661
Chicago/Turabian StyleJin, Lili, Qiuyu Wang, Hanyu Zhang, Sijia Tai, Hongsheng Liu, and Dianbao Zhang. 2018. "A Synthetic Peptide AWRK6 Alleviates Lipopolysaccharide-Induced Liver Injury" International Journal of Molecular Sciences 19, no. 9: 2661. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19092661