Lipoic Acid Prevents High-Fat Diet-Induced Hepatic Steatosis in Goto Kakizaki Rats by Reducing Oxidative Stress Through Nrf2 Activation
Abstract
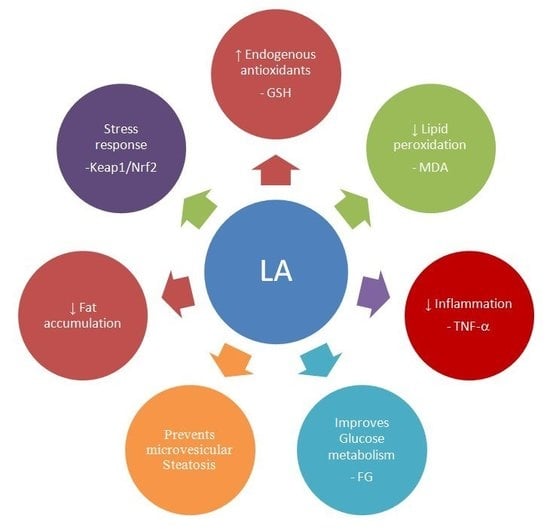
:1. Introduction
2. Results
2.1. Animal Characteristics
2.2. Histology
2.3. Intrahepatic Lipid Content
2.4. Effect of Lipoic Acid on Hepatic Antioxidant Enzymes
2.5. Oxidative Stress Biomarkers
2.6. Effect of Lipoic Acid on Liver Lipid Peroxidation
2.7. Effect of Lipoic Acid on GSH Levels
2.8. Effect of Lipoic Acid on Hepatic Nrf2 Levels
2.9. Hepatic TNF-α Levels
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Determination of Metabolic and Oxidative Stress Parameters
4.3. Lipid Content
4.4. Assay of Antioxidant Enzymes in Liver
4.5. Western Blot Analysis
4.6. Nrf2-Binding Competition Assay
4.7. Evaluation of Inflammation in the Liver
4.8. Hematoxylin/Eosin and Oil-Red O Staining
4.9. Scintigraphic Analysis
4.10. Protein
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jung, U.J.; Choi, M.S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef] [PubMed]
- Begriche, K.; Igoudjil, A.; Pessayre, D.; Fromenty, B. Mitochondrial dysfunction in NASH: Causes, consequences and possible means to prevent it. Mitochondrion 2006, 6, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yu, R.; Xiong, Y.; Du, F.; Zhu, S. A vicious circle between insulin resistance and inflammation in nonalcoholic fatty liver disease. Lipids Health Dis. 2017, 16, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C.S.; Leo, M.A.; Mak, K.M.; Xu, Y.; Cao, Q.; Ren, C.; Ponomarenko, A.; DeCarli, L.M. Model of nonalcoholic steatohepatitis. Am. J. Clin. Nutr. 2004, 79, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Marra, F.; Lotersztajn, S. Pathophysiology of NASH: Perspectives for a targeted treatment. Curr. Pharm. Des. 2013, 19, 5250–5269. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N.; Liu, J. Delaying the mitochondrial decay of aging with acetylcarnitine. Ann. N. Y. Acad. Sci. 2004, 1033, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Valdecantos, M.P.; Pérez-Matute, P.; González-Muniesa, P.; Prieto-Hontoria, P.L.; Moreno-Aliaga, M.J.; Martínez, J.A. Lipoic acid improves mitochondrial function in nonalcoholic steatosis through the stimulation of sirtuin 1 and sirtuin 3. Obesity 2012, 20, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Bird, K.E.; McMillen, T.S.; LeBoeuf, R.C.; Hagen, T.M.; Frei, B. Dietary alpha-lipoic acid supplementation inhibits atherosclerotic lesion development in apolipoprotein E-deficient and apolipoprotein E/low-density lipoprotein receptor-deficient mice. Circulation 2008, 117, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, P.; Wu, N.; He, B.; Lu, Y.; Li, S.; Liu, Y.; Zhao, S.; Liu, L.; Li, Y. Amelioration of lipid abnormalities by α-lipoic acid through antioxidative and anti-inflammatory effects. Obesity 2011, 19, 1647–1653. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, J.; Lodge, J.K.; Marcocci, L.; Tritschiler, H.J.; Packer, L.; Rihn, B.H. Lipoic acid in liver metabolism and disease. Free Radic. Biol. Med. 1998, 24, 1023–1039. [Google Scholar] [CrossRef]
- Yang, Y.; Li, W.; Liu, Y.; Sun, Y.; Li, Y.; Yao, Q.; Li, J.; Zhang, Q.; Gao, Y.; Gao, L.; et al. Alpha-lipoic acid improves high-fat diet-induced hepatic steatosis by modulating the transcription factors SREBP-1, FoxO1 and Nrf2 via the SIRT1/LKB1/AMPK pathway. J. Nutr. Biochem. 2014, 25, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, X.; Wu, W.; Wang, J.; Xie, H.; Wu, Z. Regeneration of glutathione by α-lipoic acid via Nrf2/ARE signaling pathway alleviates cadmium-induced HepG2 cell toxicity. Environ. Toxicol. Pharmacol. 2017, 51, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.T.; Tsuchia, M.; Packer, L. Thioctic acid and dihydrolipoic acid are novel antioxidants which interact with reactive oxygen species. Free Rad. Res. Commun. 1991, 15, 255–263. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Hagen, T.M.; Vinarsky, V.; Ames, B.N. Age-associated decline in ascorbic acid concentration, recycling, and biosynthesis in rat hepatocytes-reversal with (R)-α-lipoic acid supplementation. FASEB J. 1998, 12, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Sena, C.M.; Nunes, E.; Louro, T.; Proença, T.; Fernandes, R.; Boarder, M.R.; Seiça, R.M. Effects of α-lipoic acid on endothelial function in aged diabetic and high-fat fed rats. Br. J. Pharmacol. 2008, 153, 894–906. [Google Scholar] [CrossRef] [PubMed]
- Heyman, S. Hepatobiliary scintigraphy as a liver function test. J. Nucl. Med. 1994, 35, 436–437. [Google Scholar] [PubMed]
- Juni, J.E.; Reichle, R. Measurement of hepatocellular function with deconvolutional analysis: Application in the differential diagnosis of acute jaundice. Radiology 1990, 177, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Ekman, M.; Fjalling, M.; Friman, S.; Carlson, S.; Volkmann, R. Liver uptake function measured by IODIDA clearance rate in liver transplant patients and healthy volunteers. Nucl. Med. Commun. 1996, 17, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Sena, C.M.; Louro, T.; Nunes, E.; Seiça, R.M.; Proença, T.; Cipriano, M.A.; Cardoso, D.; Botelho, M.F. Liver function and plasma antioxidant status in Goto-Kakizaki diabetic rats: Effects of lipoic acid and soybean oil. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, S122. [Google Scholar]
- Sena, C.M.; Barosa, C.; Nunes, E.; Seiça, R.; Jones, J.G. Sources of endogenous glucose production in the Goto-Kakizaki diabetic rat. Diabetes Metab. 2007, 33, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Uskoković, A.; Dinić, S.; Jovanović, J.A. Liver Diseases: Epigenetic Mechanisms, Oxidative Stress and Use of Alpha-Lipoic Acid. In Handbook of Nutrition, Diet, and Epigenetics; Patel, V., Preedy, V., Eds.; Springer International Publishing: New York, NY, USA, 2017; pp. 1–21. [Google Scholar]
- Kathirvel, E.; Morgan, K.; French, S.W.; Morgan, T.R. Acetyl-L-carnitine and lipoic acid improve mitochondrial abnormalities and serum levels of liver enzymes in a mouse model of nonalcoholic fatty liver disease. Nutr. Res. 2013, 33, 932–941. [Google Scholar] [CrossRef] [PubMed]
- Shay, K.P.; Moreau, R.F.; Smith, E.J.; Smith, A.R.; Hagen, T.M. Alpha-lipoic acid as a dietary supplement: Molecular mechanisms and therapeutic potential. Biochim. Biophys. Acta 2009, 1790, 1149–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, T.S.; Kin, S.K.; Shin, H.J.; Jeon, B.T.; Hahm, J.R.; Roh, G.S. α-lipoic acid prevents non-alcoholic fatty liver disease in OLETF rats. Liver Int. 2012, 32, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A.H.; Matthan, N.R.; Jalbert, S.M.; Resteghini, N.A.; Schaefer, E.J.; Ausman, L.M. Novel soybean oils with different fatty acid profiles alter cardiovascular disease risk factors in moderately hyperlipidemic subjects. Am. J. Clin. Nutr. 2006, 84, 497–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fayez, A.M.; Zakaria, S.; Moustafa, D. Alpha lipoic acid exerts antioxidant effect via Nrf2/HO-1 pathway activation and suppresses hepatic stellate cells activation induced by methotrexate in rats. Biomed. Pharmacother. 2018, 105, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.H.; Shenvi, S.V.; Dixon, B.M.; Liu, H.; Jaiswal, A.K.; Liu, R.M.; Hagen, T.M. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc. Natl. Acad. Sci. USA 2004, 101, 3381–3386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupte, A.A.; Lyon, C.J.; Hsueh, W.A. Nuclear factor (erythroid derived 2)-like-2 factor (Nrf2), a key regulator of the antioxidant response to protect against atherosclerosis and nonalcoholic steato hepatitis. Curr. Diab. Rep. 2013, 13, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Kitteringham, N.R.; Abdullah, A.; Walsh, J.; Randle, L.; Jenkins, R.E.; Sison, R.; Goldring, C.E.; Powell, H.; Sanderson, C.; Williams, S.; et al. Proteomic analysis of Nrf2 deficient transgenic mice reveals cellular defence and lipid metabolism as primary Nrf2-dependent pathways in the liver. J. Proteomics 2010, 73, 1612–1631. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Wakabayashi, J.; Yates, M.S.; Wakabayashi, N.; Dolan, P.M.; Aja, S.; Liby, K.T.; Sporn, M.B.; Yamamoto, M.; Kensler, T.W. Role of Nrf2 in prevention of high-fat diet-induced obesity by synthetic triterpenoid CDDO-imidazolide. Eur. J. Pharmacol. 2009, 620, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Lagman, M.; Ly, J.; Saing, T.; Kaur Singh, M.; Vera Tudela, E.; Morris, D.; Chi, P.T.; Ochoa, C.; Sathananthan, A.; Venketaraman, V. Investigating the causes for decreased levels of glutathione in individuals with type 2 diabetes. PLoS ONE 2015, 10, e0118436. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Handleman, G.; Marcocci, L.; Sen, C.K.; Roy, S.; Kobuchi, H.; Tritschler, H.J.; Flohe, L.; Packer, L. Lipoic acid increases de novo synthesis of cellular glutathione by improving cysteine utilization. Biofactors 1997, 6, 321–338. [Google Scholar] [CrossRef] [PubMed]
- Kay, H.Y.; Kim, W.D.; Hwang, S.J.; Choi, H.S.; Gilroy, R.K.; Wan, Y.J.; Kim, S.G. Nrf2 inhibits LXRα-dependent hepatic lipogenesis by competing with FXR for acetylase binding. Antioxid. Redox Signal. 2011, 15, 2135–2146. [Google Scholar] [CrossRef] [PubMed]
- Bartelt, A.; Widenmaier, S.B.; Schlein, C.; Johann, K.; Goncalves, R.L.S.; Eguchi, K.; Fischer, A.W.; Parlakgül, G.; Snyder, N.A.; Nguyen, T.B.; et al. Brown adipose tissue thermogenic adaptation requires Nrf1-mediated proteasomal activity. Nat. Med. 2018, 24, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Widenmaier, S.B.; Snyder, N.A.; Nguyen, T.B.; Arduini, A.; Lee, G.Y.; Arruda, A.P.; Saksi, J.; Bartelt, A.; Hotamisligil, G.S. NRF1 Is an ER Membrane Sensor that Is Central to Cholesterol Homeostasis. Cell 2017, 171, 1094–1109. [Google Scholar] [CrossRef] [PubMed]
- Lanaspa, M.A.; Sanchez-Lozada, L.G.; Choi, Y.J.; Cicerchi, C.; Kanbay, M.; Roncal-Jimenez, C.A.; Ishimoto, T.; Li, N.; Marek, G.; Duranay, M.; et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: Potential role in fructose-dependent and -independent fatty liver. J. Biol. Chem. 2012, 287, 40732–40744. [Google Scholar] [CrossRef] [PubMed]
- Lanaspa, M.A.; Sanchez-Lozada, L.G.; Cicerchi, C.; Li, N.; Roncal-Jimenez, C.A.; Ishimoto, T.; Le, M.; Garcia, G.E.; Thomas, J.B.; Rivard, C.J.; et al. Uric acid stimulates fructokinase and accelerates fructose metabolism in the development of fatty liver. PLoS ONE 2012, 7, e47948. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Y.; Peng, C.C.; Liang, Y.J.; Yeh, W.T.; Wang, H.E.; Yu, T.H.; Peng, R.Y. Alpinia zerumbet potentially elevates high-density lipoprotein cholesterol level in hamsters. J. Agric. Food Chem. 2008, 56, 4435–4443. [Google Scholar] [CrossRef] [PubMed]
- Teschke, R.; Xuan, T.D. Viewpoint: A Contributory Role of Shell Ginger (Alpinia zerumbet (Pers.) B.L. Burtt & R.M. Sm) for Human Longevity in Okinawa, Japan? Nutrients 2018, 10, E166. [Google Scholar] [PubMed]
- Stärkel, P.; Sempoux, C.; Leclercq, I.; Herin, M.; Deby, C.; Desager, J.P.; Horsmans, Y. Oxidative stress, KLF6 and transforming growth factor-beta up-regulation differentiate non-alcoholic steatohepatitis progressing to fibrosis from uncomplicated steatosis in rats. J. Hepatol. 2003, 39, 538–546. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar]
- Hsu, C.L.; Wu, C.H.; Huang, S.L.; Yen, G.C. Phenolic compounds rutin and o-coumaric acid ameliorate obesity induced by high-fat diet in rats. J. Agric. Food Chem. 2009, 57, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.A.; Burk, R.F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Commun. 1976, 71, 952–958. [Google Scholar] [CrossRef]
- Bellomo, G.; Mirabelli, F.; DiMonte, D.; Richelmi, P.; Thor, H.; Orrenius, C.; Orrenius, S. Formation and reduction of glutathione-protein mixed disulfides during oxidative stress. Biochem. Pharmacol. 1987, 36, 1313–1320. [Google Scholar]
- Tian, J.; Lin, X.; Guan, R.; Xu, J.G. The effects of hydroxyethyl starch on lung capillary permeability in endotoxic rats and possible mechanisms. Anesth. Analg. 2004, 98, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-W.; Huang, Y.-J.; Yao, H.-T.; Lii, C.-K. Induction of Nrf2-dependent Antioxidation and Protection Against Carbon Tetrachloride-induced Liver Damage by Andrographis Herba Ethanolic Extract. J. Tradit. Complement. Med. 2012, 2, 211–219. [Google Scholar] [CrossRef]
- Bitar, M.S.; Al-Mulla, F. A defect in Nrf2 signaling constitutes a mechanism for cellular stress hypersensitivity in a genetic rat model of type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E1119–E1129. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Yen, G.C. Effect of gallic acid on high fat diet-induced dyslipidaemia, hepatosteatosis and oxidative stress in rats. Br. J. Nutr. 2007, 98, 727–735. [Google Scholar] [CrossRef] [PubMed]




| Wistar | GK | HFD | SO | LA | |
|---|---|---|---|---|---|
| Body Weight (g) | 559.7 ± 28.8 | 358.5 ± 8.7 *** | 410.9 ± 5.1 *** § | 428.8 ± 12.0 *** § | 433.9 ± 10.9 *** §§ |
| Liver Weight (g) | 6.75 ± 0.6 | 4.84 ± 0.2 * | 11.05 ± 0.2 *** §§§ | 8.32 ± 0.6 §§§ φφ | 6.37 ± 0.8 § φφφ |
| Liver Weight/Body Weight (%) | 1.19 ± 0.07 | 1.36 ± 0.19 | 2.71 ± 0.07 *** §§§ | 1.91 ± 0.11 * φφ | 1.63 ± 0.19 φφφ |
| FBG (mmol/L) | 4.07 ± 0.1 | 6.39 ± 0.4 *** | 8.42 ± 0.4 *** §§ | 7.19 ± 0.3 *** | 6.78 ± 0.3 *** φ |
| BG 2h After a Load (mmol/L) | 6.5 ± 0.6 | 18.45 ± 2.7 *** | 23.26 ± 1.6 *** | 19.9 ± 0.8 *** | 20.2 ± 0.5 *** |
| Cholesterol (mmol/L) | 2.42 ± 0.21 | 4.0 ± 0.66 | 18.27 ± 3.06 *** §§§ | 3.77 ± 0.56 φφφ | 2.39 ± 0.27 φφφ |
| Non-HDL cholesterol (mmol/L) | 0.71 ± 0.09 | 1.76 ± 0.51 | 16.01 ± 3.0 *** §§§ | 2.23 ± 0.48 φφφ | 1.11 ± 0.16 φφφ |
| Triglycerides (mmol/L) | 1.15 ± 0.16 | 1.81 ± 0.78 | 6.23 ± 0.96 *** §§§ | 2.22 ± 0.42 φφφ | 1.32 ± 0.16 φφφ |
| Wistar | GK | HFD | SO | LA | |
|---|---|---|---|---|---|
| Albumin (g/dL) | 2.7 ± 0.14 | 2.9 ± 0.1 | 2.6 ± 0.04 | 2.7 ± 0.06 | 2.4 ± 0.04 |
| T-Bilirubin (mg/dL) | 0.18 ± 0.05 | 0.16 ± 0.0 | 0.14 ± 0.02 | 0.14 ± 0.04 | 0.18 ± 0.02 |
| AST (U/L) | 147.5 ± 2.9 | 178 ± 15.1 ** | 176 ± 7.4 * | 154 ± 12.4 | 151 ± 8.7 φ |
| ALT (U/L) | 49.3 ± 3.3 | 43.6 ± 1.1 | 40.1 ± 4.3 | 27 ± 2.0 *** | 37.5 ± 1.3 *** |
| AST/ALT | 3.1 ± 0.2 | 3.9 ± 0.1 | 4.2 ± 0.2 | 5.7 ± 0.4 *** §§ φφ | 3.9 ± 0.2 ## |
| ALP (U/L) | 73.8 ± 22.1 | 137.1 ± 10.5 * | 157.3 ± 9.8 ** | 114.8 ± 7.8 φ | 125.2 ± 7.4 φ |
| γGT (U/L) | 1.5± 0.15 | 2.1±0.1 * | 1.9±0.1 * | 1.6±0.2 | 1.3±0.2 §§ φ |
| HEF (%) | 100.0 ± 1.2 | 100 ± 1.5 | 87 ± 2.9 * § | 94.9 ± 1.8 φ | 97.8 ± 1.74 φ |
| Wistar | GK | HFD | SO | LA | |
|---|---|---|---|---|---|
| GPx (nmol/min/mg protein) | 181.2 ± 3.5 | 156.4 ± 2.5 *** | 143.2 ± 2.2 *** §§ | 151.2 ± 4.5 *** φ | 183.3 ± 2.5 §§§ φφφ ### |
| GRd (nmol/min/mg protein) | 42.3 ± 2.1 | 35.2 ± 1.9 * | 28.2 ± 1.2 *** § | 32.1 ± 2.5 * | 39.5 ± 2.1 φφ |
| MDA (μmol/L) | 1.12 ± 0.1 | 1.5 ± 0.1 ** | 1.8 ± 0.1 *** | 1.42 ± 0.2 * | 1.11 ± 0.1 φφ |
| 8-OHdG (ng/24h) | 149.28 ± 17.0 | 306.57 ± 23.76 *** | 415.8 ± 74.19 *** § | 302.37 ± 42.34 ** | 5.63 ± 0.36 φφφ ### |
| Uric acid (mg/dL) | 1.2 ±0.1 | 1.6 ± 0.2 * | 1.8 ± 0.2 ** | 1.8 ± 0.2 ** | 1.2 ± 0.1 φ # |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sena, C.M.; Cipriano, M.A.; Botelho, M.F.; Seiça, R.M. Lipoic Acid Prevents High-Fat Diet-Induced Hepatic Steatosis in Goto Kakizaki Rats by Reducing Oxidative Stress Through Nrf2 Activation. Int. J. Mol. Sci. 2018, 19, 2706. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19092706
Sena CM, Cipriano MA, Botelho MF, Seiça RM. Lipoic Acid Prevents High-Fat Diet-Induced Hepatic Steatosis in Goto Kakizaki Rats by Reducing Oxidative Stress Through Nrf2 Activation. International Journal of Molecular Sciences. 2018; 19(9):2706. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19092706
Chicago/Turabian StyleSena, Cristina Maria, Maria Augusta Cipriano, Maria Filomena Botelho, and Raquel Maria Seiça. 2018. "Lipoic Acid Prevents High-Fat Diet-Induced Hepatic Steatosis in Goto Kakizaki Rats by Reducing Oxidative Stress Through Nrf2 Activation" International Journal of Molecular Sciences 19, no. 9: 2706. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19092706