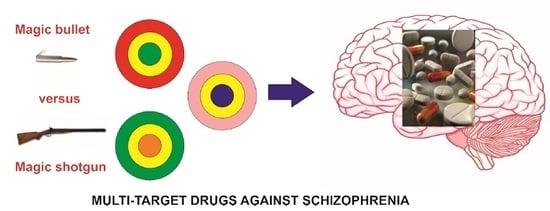
Multi-Target Approach for Drug Discovery against Schizophrenia
Abstract
:1. Introduction
2. Drug Targets for the Treatment of Schizophrenia
2.1. Dopamine and Serotonin Receptors
2.2. Adrenergic and Histaminergic Receptors
2.3. Muscarinic and Nicotinic Receptors
2.4. Metabotropic and Ionotropic Glutamatergic Receptors
2.5. Other Drug Targets in Schizophrenia
3. Multi-Target Compounds: Strategies of Design, Benefits, and Limitations
3.1. Design of Multi-Target Compounds
3.2. Advantages and Disadvantages of Multi-Target Ligands
4. Multi-Target Compounds to Treat Schizophrenia
4.1. Marketed Drugs—Second and Third Generation Antipsychotics
4.2. Other Multi-Target Compounds for the Treatment of Schizophrenia
4.2.1. Modifications of Marketed Drugs
4.2.2. Other Multi-Target Compounds with Potential Application for the Treatment of Schizophrenia
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 3D QSAR | Three-dimensional structure-activity relationship |
| AMPA | α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| cAMP | Cyclic adenosine monophosphate |
| CAR | Conditioned avoidance response |
| CNS | Central nervous system |
| cNT PDEs | Cyclic nucleotide phosphodiestereases |
| EPS | Extrapyramidal symptoms |
| FDA | Food and Drug Administration |
| GABA | γ-Aminobutyric acid |
| GPCRs | G protein-coupled receptors |
| GSK-3 | Glycogen synthase kinase-3 |
| GTP | Guanosine-5′-triphosphate |
| LSD | Lysergic acid diethylamide |
| MTDs | Multi-target drugs |
| NMDA | N-methyl-d-aspartate |
| PCP | Phencyclidine |
| SSRI | Selective serotonin reuptake inhibitor |
| VTA | Ventral tegmental area |
References
- Stępnicki, P.; Kondej, M.; Kaczor, A.A. Current Concepts and Treatments of Schizophrenia. Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.-I.; Wang, H.-C.; Hsu, J.-L.; Liu, M.-E. Does the dopamine hypothesis explain schizophrenia? Rev. Neurosci. 2013, 24, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; MacDonald, M.L.; Elswick, D.E.; Sweet, R.A. The glutamate hypothesis of schizophrenia: Evidence from human brain tissue studies. Ann. N. Y. Acad. Sci. 2015, 1338, 38–57. [Google Scholar] [CrossRef] [PubMed]
- Lavecchia, A.; Cerchia, C. In silico methods to address polypharmacology: Current status, applications and future perspectives. Drug Discov. Today 2016, 21, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Korcsmáros, T.; Szalay, M.S.; Böde, C.; Kovács, I.A.; Csermely, P. How to design multi-target drugs. Expert Opin. Drug Discov. 2007, 2, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, K.; Mavridis, L.; Djikic, T.; Vucicevic, J.; Agbaba, D.; Yelekci, K.; Mitchell, J.B.O. Drug Design for CNS Diseases: Polypharmacological Profiling of Compounds Using Cheminformatic, 3D-QSAR and Virtual Screening Methodologies. Front. Neurosci. 2016, 10, 265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakhshi, K.; Chance, S.A. The neuropathology of schizophrenia: A selective review of past studies and emerging themes in brain structure and cytoarchitecture. Neuroscience 2015, 303, 82–102. [Google Scholar] [CrossRef] [PubMed]
- Rang, H.P.; Ritter, J.M.; Flower, R.J.; Henderson, G. Rang and Dale’s Pharmacology, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Miyamoto, S.; Duncan, G.E.; Marx, C.E.; Lieberman, J.A. Treatments for schizophrenia: A critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol. Psychiatry 2005, 10, 79–104. [Google Scholar] [CrossRef] [PubMed]
- Mauri, M.C.; Paletta, S.; Maffini, M.; Colasanti, A.; Dragogna, F.; di Pace, C.; Altamura, A.C. Clinical pharmacology of atypical antipsychotics: An update. EXCLI J. 2014, 13, 1163–1191. [Google Scholar] [PubMed]
- Orsolini, L.; Tomasetti, C.; Valchera, A.; Vecchiotti, R.; Matarazzo, I.; Vellante, F.; Iasevoli, F.; Buonaguro, E.F.; Fornaro, M.; Fiengo, A.L.C.; et al. An update of safety of clinically used atypical antipsychotics. Expert Opin. Drug Saf. 2016, 15, 1329–1347. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, H.Y.; Nash, J.F. Effects of antipsychotic drugs on serotonin receptors. Pharmacol. Rev. 1991, 43, 587–604. [Google Scholar] [PubMed]
- Sorensen, S.M.; Kehne, J.H.; Fadayel, G.M.; Humphreys, T.M.; Ketteler, H.J.; Sullivan, C.K.; Taylor, V.L.; Schmidt, C.J. Characterization of the 5-HT2 receptor antagonist MDL 100907 as a putative atypical antipsychotic: Behavioral, electrophysiological and neurochemical studies. J. Pharmacol. Exp. Ther. 1993, 266, 684–691. [Google Scholar] [PubMed]
- Miller, C.H.; Fleischhacker, W.W.; Ehrmann, H.; Kane, J.M. Treatment of neuroleptic induced akathisia with the 5-HT2 antagonist ritanserin. Psychopharmacol. Bull. 1990, 26, 373–376. [Google Scholar] [PubMed]
- Schmidt, C.J.; Sorensen, S.M.; Kehne, J.H.; Carr, A.A.; Palfreyman, M.G. The role of 5-HT2A receptors in antipsychotic activity. Life Sci. 1995, 56, 2209–2222. [Google Scholar] [CrossRef]
- Meltzer, H.Y.; Li, Z.; Kaneda, Y.; Ichikawa, J. Serotonin receptors: Their key role in drugs to treat schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2003, 27, 1159–1172. [Google Scholar] [CrossRef] [PubMed]
- Millan, M.J. Improving the treatment of schizophrenia: Focus on serotonin 5-HT1A receptors. J. Pharmacol. Exp. Ther. 2000, 295, 853–861. [Google Scholar] [PubMed]
- Akimova, E.; Lanzenberger, R.; Kasper, S. The serotonin-1A receptor in anxiety disorders. Biol. Psychiatry 2009, 66, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Ogren, S.O.; Eriksson, T.M.; Elvander-Tottie, E.; D’Addario, C.; Ekström, J.C.; Svenningsson, P.; Meister, B.; Kehr, J.; Stiedl, O. The role of 5-HT1A receptors in learning and memory. Behav. Brain Res. 2008, 195, 54–77. [Google Scholar] [CrossRef] [PubMed]
- Prinssen, E.P.; Colpaert, F.C.; Koek, W. 5-HT1A receptor activation and anti-cataleptic effects: High-efficacy agonists maximally inhibit haloperidol-induced catalepsy. Eur. J. Pharmacol. 2002, 453, 217–221. [Google Scholar] [CrossRef]
- Mignon, L.; Wolf, W.A. Postsynaptic 5-HT1A receptors mediate an increase in locomotor activity in the monoamine-depleted rat. Psychopharmacology 2002, 163, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Madjid, N.; Tottie, E.E.; Lüttgen, M.; Meister, B.; Sandin, J.; Kuzmin, A.; Stiedl, O.; Ogren, S.O. 5-Hydroxytryptamine 1A receptor blockade facilitates aversive learning in mice: Interactions with cholinergic and glutamatergic mechanisms. J. Pharmacol. Exp. Ther. 2006, 316, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y. Therapeutic role of 5-HT1A receptors in the treatment of schizophrenia and Parkinson’s disease. CNS Neurosci. Ther. 2011, 17, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Kennett, G.A.; Curzon, G. Evidence that mCPP may have behavioural effects mediated by central 5-HT1C receptors. Br. J. Pharmacol. 1988, 94, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Kalkman, H.O. Hypersensitivity to meta-chlorophenylpiperazine (mCPP) in migraine and drug withdrawal. Int. J. Clin. Pharmacol. Res. 1997, 17, 75–77. [Google Scholar] [PubMed]
- Krystal, J.H.; Seibyl, J.P.; Price, L.H.; Woods, S.W.; Heninger, G.R.; Aghajanian, G.K.; Charney, D.S. m-Chlorophenylpiperazine effects in neuroleptic-free schizophrenic patients. Evidence implicating serotonergic systems in the positive symptoms of schizophrenia. Arch. Gen. Psychiatry 1993, 50, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.D.; Heidbreder, C.; Reavill, C.; Ashby, C.R., Jr.; Middlemiss, D.N. 5-HT2C receptor antagonists: Potential in schizophrenia. Drug Dev. Res. 2001, 54, 88–94. [Google Scholar]
- Foster, D.J.; Conn, P.J. Allosteric Modulation of GPCRs: New Insights and Potential Utility for Treatment of Schizophrenia and Other CNS Disorders. Neuron 2017, 94, 431–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urs, N.M.; Gee, S.M.; Pack, T.F.; McCorvy, J.D.; Evron, T.; Snyder, J.C.; Yang, X.; Rodriguiz, R.M.; Borrelli, E.; Wetsel, W.C.; et al. Distinct cortical and striatal actions of a β-arrestin-biased dopamine D2 receptor ligand reveal unique antipsychotic-like properties. Proc. Natl. Acad. Sci. USA 2016, 113, E8178–E8186. [Google Scholar] [CrossRef] [PubMed]
- Kaczor, A.A.; Selent, J. Oligomerization of G protein-coupled receptors: Biochemical and biophysical methods. Curr. Med. Chem. 2011, 18, 4606–4634. [Google Scholar] [CrossRef] [PubMed]
- Selent, J.; Kaczor, A.A. Oligomerization of G protein-coupled receptors: Computational methods. Curr. Med. Chem. 2011, 18, 4588–4605. [Google Scholar] [CrossRef] [PubMed]
- Kaczor, A.A.; Jörg, M.; Capuano, B. The dopamine D2 receptor dimer and its interaction with homobivalent antagonists: Homology modeling, docking and molecular dynamics. J. Mol. Model. 2016, 22, 203. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.L.; Miranda-Azpiazu, P.; García-Bea, A.; Younkin, J.; Cui, M.; Kozlenkov, A.; Ben-Ezra, A.; Voloudakis, G.; Fakira, A.K.; Baki, L.; et al. Allosteric signaling through an mGlu2 and 5-HT2A heteromeric receptor complex and its potential contribution to schizophrenia. Sci. Signal. 2016, 9, ra5. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.H.F.; Tarazi, F.I.; Shahid, M. The effectiveness of multi-target agents in schizophrenia and mood disorders: Relevance of receptor signature to clinical action. Pharmacol. Ther. 2010, 126, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Maletic, V.; Eramo, A.; Gwin, K.; Offord, S.J.; Duffy, R.A. The Role of Norepinephrine and Its α-Adrenergic Receptors in the Pathophysiology and Treatment of Major Depressive Disorder and Schizophrenia: A. Systematic Review. Front. Psychiatry 2017, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Svensson, T.H. α-Adrenoceptor modulation hypothesis of antipsychotic atypicality. Prog. Neuropsychopharmacol. Biol. Psychiatry 2003, 27, 1145–1158. [Google Scholar] [CrossRef] [PubMed]
- Brosda, J.; Jantschak, F.; Pertz, H.H. α2-Adrenoceptors are targets for antipsychotic drugs. Psychopharmacology 2014, 231, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Marcus, M.M.; Wiker, C.; Frånberg, O.; Konradsson-Geuken, A.; Langlois, X.; Jardemark, K.; Svensson, T.H. Adjunctive α2-adrenoceptor blockade enhances the antipsychotic-like effect of risperidone and facilitates cortical dopaminergic and glutamatergic, NMDA receptor-mediated transmission. Int. J. Neuropsychopharmacol. 2010, 13, 891–903. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Deng, C.; Huang, X.-F. The role of hypothalamic H1 receptor antagonism in antipsychotic-induced weight gain. CNS Drugs 2013, 27, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Ellenbroek, B.A.; Ghiabi, B. Do Histamine receptor 3 antagonists have a place in the therapy for schizophrenia? Curr. Pharm. Des. 2015, 21, 3760–3770. [Google Scholar] [CrossRef] [PubMed]
- Sadek, B.; Saad, A.; Sadeq, A.; Jalal, F.; Stark, H. Histamine H3 receptor as a potential target for cognitive symptoms in neuropsychiatric diseases. Behav. Brain Res. 2016, 312, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, A.; Rook, J.M.; Dickerson, J.W.; Roop, G.N.; Morrison, R.D.; Jalan-Sakrikar, N.; Lamsal, A.; Noetzel, M.J.; Poslusney, M.S.; Wood, M.R.; et al. Potentiation of M1 Muscarinic Receptor Reverses Plasticity Deficits and Negative and Cognitive Symptoms in a Schizophrenia Mouse Model. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2016, 41, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Dean, B.; Scarr, E. Possible involvement of muscarinic receptors in psychiatric disorders: A focus on schizophrenia and mood disorders. Curr. Mol. Med. 2015, 15, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Holt, D.J.; Bachus, S.E.; Hyde, T.M.; Wittie, M.; Herman, M.M.; Vangel, M.; Saper, C.B.; Kleinman, J.E. Reduced density of cholinergic interneurons in the ventral striatum in schizophrenia: An in situ hybridization study. Biol. Psychiatry 2005, 58, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Raedler, T.J.; Knable, M.B.; Jones, D.W.; Urbina, R.A.; Gorey, J.G.; Lee, K.S.; Egan, M.F.; Coppola, R.; Weinberger, D.R. In vivo determination of muscarinic acetylcholine receptor availability in schizophrenia. Am. J. Psychiatry 2003, 160, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Raedler, T.J.; Bymaster, F.P.; Tandon, R.; Copolov, D.; Dean, B. Towards a muscarinic hypothesis of schizophrenia. Mol. Psychiatry 2007, 12, 232–246. [Google Scholar] [CrossRef] [PubMed]
- Freeman, T.P.; Stone, J.M.; Orgaz, B.; Noronha, L.A.; Minchin, S.L.; Curran, H.V. Tobacco smoking in schizophrenia: Investigating the role of incentive salience. Psychol. Med. 2014, 44, 2189–2197. [Google Scholar] [CrossRef] [PubMed]
- Parikh, V.; Kutlu, M.G.; Gould, T.J. nAChR dysfunction as a common substrate for schizophrenia and comorbid nicotine addiction: Current trends and perspectives. Schizophr. Res. 2016, 171, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, C.E.; Stevens, K.E. Evidence for a role of nicotinic acetylcholine receptors in schizophrenia. Front. Biosci. J. Virtual Libr. 2007, 12, 4755–4772. [Google Scholar] [CrossRef]
- Li, Y.; Sun, L.; Yang, T.; Jiao, W.; Tang, J.; Huang, X.; Huang, Z.; Meng, Y.; Luo, L.; Wang, X.; et al. Design and Synthesis of Novel Positive Allosteric Modulators of α7 Nicotinic Acetylcholine Receptors with the Ability To Rescue Auditory Gating Deficit in Mice. J. Med. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Neves, G.A.; Grace, A.A. α7 Nicotinic receptor-modulating agents reverse the hyperdopaminergic tone in the MAM model of schizophrenia. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2018, 43, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, B.; Javitt, D. From Revolution to Evolution: The Glutamate Hypothesis of Schizophrenia and its Implication for Treatment. Neuropsychopharmacology 2012, 37, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.C.; Tsai, S.-J. New Targets for Schizophrenia Treatment beyond the Dopamine Hypothesis. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Goff, D.C.; Coyle, J.T. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am. J. Psychiatry 2001, 158, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.J.; Weinberger, D.R. Schizophrenia genes, gene expression, and neuropathology: On the matter of their convergence. Mol. Psychiatry 2005, 10, 40–68. [Google Scholar] [CrossRef] [PubMed]
- Cull-Candy, S.; Brickley, S.; Farrant, M. NMDA receptor subunits: Diversity, development and disease. Curr. Opin. Neurobiol. 2001, 11, 327–335. [Google Scholar] [CrossRef]
- Paoletti, P.; Neyton, J. NMDA receptor subunits: Function and pharmacology. Curr. Opin. Pharmacol. 2007, 7, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S.; Shaukat, F.; Gul, A.; Arooj, M.; Malik, A. Potential role of amino acids in pathogenesis of schizophrenia. Int. J. Health Sci. (Quassim) 2017, 11, 63–68. [Google Scholar]
- Farber, N.B. The NMDA receptor hypofunction model of psychosis. Ann. N. Y. Acad. Sci. 2003, 1003, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Goff, D.C. Glutamate receptors in schizophrenia and antipsychotic drugs, in Neurotransmitter Receptors. In Actions of Antipsychotic Medications; Lidow, M.S., Ed.; CRC Press: New York, NY, USA, 2000; pp. 121–136. [Google Scholar]
- Meador-Woodruff, J.H.; Healy, D.J. Glutamate receptor expression in schizophrenic brain. Brain Res. Brain Res. Rev. 2000, 31, 288–294. [Google Scholar] [CrossRef]
- Gao, X.M.; Sakai, K.; Roberts, R.C.; Conley, R.R.; Dean, B.; Tamminga, C.A. Ionotropic glutamate receptors and expression of N-methyl-d-aspartate receptor subunits in subregions of human hippocampus: Effects of schizophrenia. Am. J. Psychiatry 2000, 157, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.M.; Hogg, A.J.; Healy, D.J.; Haroutunian, V.; Davis, K.L.; Meador-Woodruff, J.H. Ionotropic glutamate receptor binding and subunit mRNA expression in thalamic nuclei in schizophrenia. Am. J. Psychiatry 2000, 157, 1811–1823. [Google Scholar] [CrossRef] [PubMed]
- Heckers, S.; Goff, D.; Schacter, D.L.; Savage, C.R.; Fischman, A.J.; Alpert, N.M.; Rauch, S.L. Functional imaging of memory retrieval in deficit vs nondeficit schizophrenia. Arch. Gen. Psychiatry 1999, 56, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Kondziella, D.; Brenner, E.; Eyjolfsson, E.M.; Sonnewald, U. How do glial–neuronal interactions fit into current neurotransmitter hypotheses of schizophrenia? Neurochem. Int. 2007, 50, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.M.; Morrison, P.D.; Pilowsky, L.S. Glutamate and dopamine dysregulation in schizophrenia—A synthesis and selective review. J. Psychopharmacol. Oxf. Engl. 2007, 21, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, L.G.; Schuff, N.; Cashdollar, N.; Weiner, M.W. Age-related glutamate and glutamine concentration changes in normal human brain: 1H MR spectroscopy study at 4 T. Neurobiol. Aging 2005, 26, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Leveque, J.C.; Macías, W.; Rajadhyaksha, A.; Carlson, R.R.; Barczak, A.; Kang, S.; Li, X.M.; Coyle, J.T.; Huganir, R.L.; Heckers, S.; et al. Intracellular modulation of NMDA receptor function by antipsychotic drugs. J. Neurosci. Off. J. Soc. Neurosci. 2000, 20, 4011–4020. [Google Scholar] [CrossRef]
- Arvanov, V.L.; Liang, X.; Schwartz, J.; Grossman, S.; Wang, R.Y. Clozapine and haloperidol modulate N-methyl-d-aspartate- and non-N-methyl-d-aspartate receptor-mediated neurotransmission in rat prefrontal cortical neurons in vitro. J. Pharmacol. Exp. Ther. 1997, 283, 226–234. [Google Scholar] [PubMed]
- Hashimoto, K.; Malchow, B.; Falkai, P.; Schmitt, A. Glutamate modulators as potential therapeutic drugs in schizophrenia and affective disorders. Eur. Arch. Psychiatry Clin. Neurosci. 2013, 263, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Menniti, F.S.; Lindsley, C.W.; Conn, P.J.; Pandit, J.; Zagouras, P.; Volkmann, R.A. Allosteric modulators for the treatment of schizophrenia: Targeting glutamatergic networks. Curr. Top. Med. Chem. 2013, 13, 26–54. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.E.; Pennicott, L.E.; Beswick, P. AMPA receptor-positive allosteric modulators for the treatment of schizophrenia: An overview of recent patent applications. Future Med. Chem. 2015, 7, 473–491. [Google Scholar] [CrossRef] [PubMed]
- Herman, E.J.; Bubser, M.; Conn, P.J.; Jones, C.K. Metabotropic glutamate receptors for new treatments in schizophrenia. Handb. Exp. Pharmacol. 2012, 297–365. [Google Scholar] [CrossRef]
- Li, M.-L.; Hu, X.-Q.; Li, F.; Gao, W.-J. Perspectives on the mGluR2/3 agonists as a therapeutic target for schizophrenia: Still promising or a dead end? Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 60, 66–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Leary, O.; Nolan, Y. Glycogen synthase kinase-3 as a therapeutic target for cognitive dysfunction in neuropsychiatric disorders. CNS Drugs 2015, 29, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Snyder, G.L.; Vanover, K.E. PDE Inhibitors for the Treatment of Schizophrenia. Adv. Neurobiol. 2017, 17, 385–409. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Kour, K.; Jayaram, M.B. Acetylcholinesterase inhibitors for schizophrenia. Cochrane Database Syst. Rev. 2012, 1, CD007967. [Google Scholar] [CrossRef] [PubMed]
- Medina-Franco, J.L.; Giulianotti, M.A.; Welmaker, G.S.; Houghten, R.A. Shifting from the single to the multitarget paradigm in drug discovery. Drug Discov. Today 2013, 18, 495–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, H.; Fridkin, M.; Youdim, M. From Single Target to Multitarget/Network Therapeutics in Alzheimer’s Therapy. Pharmaceuticals 2014, 7, 113–135. [Google Scholar] [CrossRef] [PubMed]
- Bansal, Y.; Silakari, O. Multifunctional compounds: Smart molecules for multifactorial diseases. Eur. J. Med. Chem. 2014, 76, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Lucio, O.; Naveja, J.J.; Vite-Caritino, H.; Prieto-Martínez, F.D.; Medina-Franco, J.L.; Méndez-Lucio, O.; Naveja, J.J.; Vite-Caritino, H.; Prieto-Martínez, F.D.; Medina-Franco, J.L. Review. One Drug for Multiple Targets: A Computational Perspective. J. Mex. Chem. Soc. 2016, 60, 168–181. [Google Scholar]
- Talevi, A. Multi-target pharmacology: Possibilities and limitations of the “skeleton key approach” from a medicinal chemist perspective. Front. Pharmacol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Wichur, T.; Malawska, B. Multifunctional ligands—A new approach in the search for drugs against multi-factorial diseases. Postepy Hig. Med. Doswiadczalnej Online 2015, 69, 1423–1434. [Google Scholar]
- Morphy, R.; Rankovic, Z. Designed Multiple Ligands. An Emerging Drug Discovery Paradigm. J. Med. Chem. 2005, 48, 6523–6543. [Google Scholar] [CrossRef] [PubMed]
- Kaczor, A.A.; Silva, A.G.; Loza, M.I.; Kolb, P.; Castro, M.; Poso, A. Structure-Based Virtual Screening for Dopamine D2 Receptor Ligands as Potential Antipsychotics. ChemMedChem 2016, 11, 718–729. [Google Scholar] [CrossRef] [PubMed]
- Bawa, P.; Pradeep, P.; Kumar, P.; Choonara, Y.E.; Modi, G.; Pillay, V. Multi-target therapeutics for neuropsychiatric and neurodegenerative disorders. Drug Discov. Today 2016, 21, 1886–1914. [Google Scholar] [CrossRef] [PubMed]
- Roth, B.L.; Sheffler, D.J.; Kroeze, W.K. Magic shotguns versus magic bullets: Selectively non-selective drugs for mood disorders and schizophrenia. Nat. Rev. Drug Discov. 2004, 3, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Naheed, M.; Green, B. Focus on Clozapine. Curr. Med. Res. Opin. 2001, 17, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Saklad, S.R. Graphic representation of pharmacology: Development of an alternative model. Ment. Health Clin. 2018, 7, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Boyda, H.N.; Ramos-Miguel, A.; Procyshyn, R.M.; Töpfer, E.; Lant, N.; Choy, H.H.T.; Wong, R.; Li, L.; Pang, C.C.Y.; Honer, W.G.; et al. Routine exercise ameliorates the metabolic side-effects of treatment with the atypical antipsychotic drug olanzapine in rats. Int. J. Neuropsychopharmacol. 2014, 17, 77–90. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Zhang, Q.; Deng, C.; Wang, H.; Lian, J.; Huang, X.-F. Hypothalamic histamine H1 receptor-AMPK signaling time-dependently mediates olanzapine-induced hyperphagia and weight gain in female rats. Psychoneuroendocrinology 2014, 42, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Godlewska, B.R.; Olajossy-Hilkesberger, L.; Ciwoniuk, M.; Olajossy, M.; Marmurowska-Michałowska, H.; Limon, J.; Landowski, J. Olanzapine-induced weight gain is associated with the -759C/T and -697G/C polymorphisms of the HTR2C gene. Pharmacogenomics J. 2009, 9, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, C.M.; Pilowsky, L.S. Psychopharmacology of olanzapine. A. review. Br. J. Psychiatry Suppl. 1999, 38, 52–58. [Google Scholar] [CrossRef]
- Selent, J.; López, L.; Sanz, F.; Pastor, M. Multi-receptor binding profile of clozapine and olanzapine: A structural study based on the new β2 adrenergic receptor template. ChemMedChem 2008, 3, 1194–1198. [Google Scholar] [CrossRef] [PubMed]
- Piróg-Balcerzak, A.; Habrat, B.; Mierzejewski, P. Misuse and abuse of quetiapine. Psychiatr. Pol. 2015, 49, 81–93. [Google Scholar] [CrossRef] [PubMed]
- George, B.; Craig, S. Brenner and Stevens’ Pharmacology, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Hasnain, M.; Vieweg, W.V.R. Weight considerations in psychotropic drug prescribing and switching. Postgrad. Med. 2013, 125, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Fornaro, M.; de Berardis, D.; Perna, G.; Solmi, M.; Veronese, N.; Orsolini, L.; Buonaguro, E.F.; Iasevoli, F.; Köhler, C.A.; Carvalho, A.F.; et al. Lurasidone in the Treatment of Bipolar Depression: Systematic Review of Systematic Reviews. BioMed Res. Int. 2017, 2017, 3084859. [Google Scholar] [CrossRef] [PubMed]
- Urs, N.M.; Peterson, S.M.; Caron, M.G. New Concepts in Dopamine D2 Receptor Biased Signaling and Implications for Schizophrenia Therapy. Biol. Psychiatry 2017, 81, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Brust, T.F.; Hayes, M.P.; Roman, D.L.; Watts, V.J. New functional activity of aripiprazole revealed. Robust antagonism of D2 dopamine receptor stimulated by Gβγ antagonism. Biochem Pharmacol. 2015, 93, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Tamminga, C.A. Partial dopamine agonists in the treatment of psychosis. J. Neural Transm. Vienna Austria 1996 2002, 109, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Mailman, R.B.; Murthy, V. Third generation antipsychotic drugs: Partial agonism or receptor functional selectivity? Curr. Pharm. Des. 2010, 16, 488–501. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, J.A. Dopamine partial agonists: A new class of antipsychotic. CNS Drugs 2004, 18, 251–267. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.A.; Yost, J.M.; Setola, V.; Chen, X.; Sassano, M.F.; Chen, M.; Peterson, S.; Yadav, P.N.; Huang, X.; Feng, B.; et al. Discovery of β-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc. Natl. Acad. Sci. USA 2011, 108, 18488–18493. [Google Scholar] [CrossRef] [PubMed]
- Burris, K.D.; Molski, T.F.; Xu, C.; Ryan, E.; Tottori, K.; Kikuchi, T.; Yocca, F.D.; Molinoff, P.B. Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J. Pharmacol. Exp. Ther. 2002, 302, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, D.A.; Renock, S.; Arrington, E.; Chiodo, L.A.; Liu, L.-X.; Sibley, D.R.; Roth, B.L.; Mailman, R. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2003, 28, 1400–1411. [Google Scholar] [CrossRef] [PubMed]
- Lawler, C.P.; Prioleau, C.; Lewis, M.M.; Mak, C.; Jiang, D.; Schetz, J.A.; Gonzalez, A.M.; Sibley, D.R.; Mailman, R.B. Interactions of the novel antipsychotic aripiprazole (OPC-14597) with dopamine and serotonin receptor subtypes. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 1999, 20, 612–627. [Google Scholar] [CrossRef]
- Fell, M.J.; Perry, K.W.; Falcone, J.F.; Johnson, B.G.; Barth, V.N.; Rash, K.S.; Lucaites, V.L.; Threlkeld, P.G.; Monn, J.A.; McKinzie, D.L.; et al. In vitro and in vivo evidence for a lack of interaction with dopamine D2 receptors by the metabotropic glutamate 2/3 receptor agonists 1S,2S,5R,6S-2-aminobicyclo[3.1.0]hexane-2,6-bicaroxylate monohydrate (LY354740) and (−)-2-oxa-4-aminobicyclo[3.1.0] Hexane-4,6-dicarboxylic acid (LY379268). J. Pharmacol. Exp. Ther. 2009, 331, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Masri, B.; Salahpour, A.; Didriksen, M.; Ghisi, V.; Beaulieu, J.-M.; Gainetdinov, R.R.; Caron, M.G. Antagonism of dopamine D2 receptor/β-arrestin 2 interaction is a common property of clinically effective antipsychotics. Proc. Natl. Acad. Sci. USA 2008, 105, 13656–13661. [Google Scholar] [CrossRef] [PubMed]
- Klewe, I.V.; Nielsen, S.M.; Tarpø, L.; Urizar, E.; Dipace, C.; Javitch, J.A.; Gether, U.; Egebjerg, J.; Christensen, K.V. Recruitment of β-arrestin2 to the dopamine D2 receptor: Insights into anti-psychotic and anti-parkinsonian drug receptor signaling. Neuropharmacology 2008, 54, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, E.L.A.; de Mendonça Lima, T.; Vieira, M.E.B.; Storpirtis, S.; Aguiar, P.M. Efficacy and safety of aripiprazole for the treatment of schizophrenia: An overview of systematic reviews. Eur. J. Clin. Pharmacol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Leucht, S.; Cipriani, A.; Spineli, L.; Mavridis, D.; Orey, D.; Richter, F.; Samara, M.; Barbui, C.; Engel, R.R.; Geddes, J.R.; et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: A multiple-treatments meta-analysis. Lancet Lond. Engl. 2013, 382, 951–962. [Google Scholar] [CrossRef]
- Diefenderfer, L.A.; Iuppa, C. Brexpiprazole: A review of a new treatment option for schizophrenia and major depressive disorder. Ment. Health Clin. 2017, 7, 207–212. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, J.; Citrome, L. Brexpiprazole for the Treatment of Schizophrenia: A Review of this Novel Serotonin-Dopamine Activity Modulator. Clin. Schizophr. Relat. Psychoses 2016, 9, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.H.; Diduch, M.; Gardner, K.N.; Thomas, C. Review of cariprazine in management of psychiatric illness. Ment. Health Clin. 2017, 7, 221–229. [Google Scholar] [CrossRef] [PubMed]
- De Berardis, D.; Orsolini, L.; Iasevoli, F.; Prinzivalli, E.; de Bartolomeis, A.; Serroni, N.; Mazza, M.; Valchera, A.; Fornaro, M.; Vecchiotti, R.; et al. The Novel Antipsychotic Cariprazine (RGH-188): State-of-the-Art in the Treatment of Psychiatric Disorders. Curr. Pharm. Des. 2016, 22, 5144–5162. [Google Scholar] [CrossRef] [PubMed]
- Marciniec, K.; Kurczab, R.; Książek, M.; Bębenek, E.; Chrobak, E.; Satała, G.; Bojarski, A.J.; Kusz, J.; Zajdel, P. Structural determinants influencing halogen bonding: A case study on azinesulfonamide analogs of aripiprazole as 5-HT1A, 5-HT7, and D2 receptor ligands. Chem. Cent. J. 2018, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Zajdel, P.; Marciniec, K.; Maślankiewicz, A.; Grychowska, K.; Satała, G.; Duszyńska, B.; Lenda, T.; Siwek, A.; Nowak, G.; Partyka, A.; et al. Antidepressant and antipsychotic activity of new quinoline- and isoquinoline-sulfonamide analogs of aripiprazole targeting serotonin 5-HT1A/5-HT2A/5-HT7 and dopamine D2/D3 receptors. Eur. J. Med. Chem. 2013, 60, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Butini, S.; Gemma, S.; Campiani, G.; Franceschini, S.; Trotta, F.; Borriello, M.; Ceres, N.; Ros, S.; Coccone, S.S.; Bernetti, M.; et al. Discovery of a New Class of Potential Multifunctional Atypical Antipsychotic Agents Targeting Dopamine D3 and Serotonin 5-HT1A and 5-HT2A Receptors: Design, Synthesis, and Effects on Behavior. J. Med. Chem. 2009, 52, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Butini, S.; Campiani, G.; Franceschini, S.; Trotta, F.; Kumar, V.; Guarino, E.; Borrelli, G.; Fiorini, I.; Novellino, E.; Fattorusso, C.; et al. Discovery of bishomo(hetero)arylpiperazines as novel multifunctional ligands targeting dopamine D3 and serotonin 5-HT1A and 5-HT2A receptors. J. Med. Chem. 2010, 53, 4803–4807. [Google Scholar] [CrossRef] [PubMed]
- Zajdel, P.; Marciniec, K.; Maślankiewicz, A.; Satała, G.; Duszyńska, B.; Bojarski, A.J.; Partyka, A.; Jastrzbska-Wisek, M.; Wróbel, D.; Wesołowska, A.; et al. Quinoline- and isoquinoline-sulfonamide derivatives of LCAP as potent CNS multi-receptor—5-HT1A/5-HT2A/5-HT7 and D2/D3/D4—Agents: The synthesis and pharmacological evaluation. Bioorg. Med. Chem. 2012, 20, 1545–1556. [Google Scholar] [CrossRef] [PubMed]
- Partyka, A.; Kurczab, R.; Canale, V.; Satała, G.; Marciniec, K.; Pasierb, A.; Jastrzębska-Więsek, M.; Pawłowski, M.; Wesołowska, A.; Bojarski, A.J.; et al. The impact of the halogen bonding on D2 and 5-HT1A/5-HT7 receptor activity of azinesulfonamides of 4-[(2-ethyl)piperidinyl-1-yl]phenylpiperazines with antipsychotic and antidepressant properties. Bioorg. Med. Chem. 2017, 25, 3638–3648. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, S.H.; Kanhed, A.M.; Dash, R.C.; Suryawanshi, M.R.; Mahadik, K.R. Design, synthesis, pharmacological evaluation and computational studies of 1-(biphenyl-4-yl)-2-[4-(substituted phenyl)-piperazin-1-yl]ethanones as potential antipsychotics. Eur. J. Med. Chem. 2014, 74, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, H.G.; Dapper, E.A.; Vinkers, C.H. SSRIs and depressive symptoms in schizophrenia: A. systematic review. Tijdschr. Voor Psychiatr. 2017, 59, 40–46. [Google Scholar]
- Van Hes, R.; Smid, P.; Stroomer, C.N.J.; Tipker, K.; Tulp, M.T.M.; van der Heyden, J.A.M.; McCreary, A.C.; Hesselink, M.B.; Kruse, C.G. SLV310, a novel, potential antipsychotic, combining potent dopamine D2 receptor antagonism with serotonin reuptake inhibition. Bioorg. Med. Chem. Lett. 2003, 13, 405–408. [Google Scholar] [CrossRef]
- Smid, P.; Coolen, H.K.A.C.; Keizer, H.G.; van Hes, R.; de Mos, J.-P.; den Hartog, A.P.; Stork, B.; Plekkenpol, R.H.; Niemann, L.C.; Stroomer, C.N.J.; et al. Synthesis, structure-activity relationships, and biological properties of 1-heteroaryl-4-[ω-(1H-indol-3-yl)alkyl]piperazines, novel potential antipsychotics combining potent dopamine D2 receptor antagonism with potent serotonin reuptake inhibition. J. Med. Chem. 2005, 48, 6855–6869. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, Q.; Robichaud, A.J.; Lee, T.; Tomesch, J.; Yao, W.; Beard, J.D.; Snyder, G.L.; Zhu, H.; Peng, Y.; et al. Discovery of a Tetracyclic Quinoxaline Derivative as a Potent and Orally Active Multifunctional Drug Candidate for the Treatment of Neuropsychiatric and Neurological Disorders. J. Med. Chem. 2014, 57, 2670–2682. [Google Scholar] [CrossRef] [PubMed]
- Zajdel, P.; Kos, T.; Marciniec, K.; Satała, G.; Canale, V.; Kamiński, K.; Hołuj, M.; Lenda, T.; Koralewski, R.; Bednarski, M.; et al. Novel multi-target azinesulfonamides of cyclic amine derivatives as potential antipsychotics with pro-social and pro-cognitive effects. Eur. J. Med. Chem. 2018, 145, 790–804. [Google Scholar] [CrossRef] [PubMed]
- Menegatti, R.; Cunha, A.C.; Ferreira, V.F.; Perreira, E.F.; El-Nabawi, A.; Eldefrawi, A.T.; Albuquerque, E.X.; Neves, G.; Rates, S.M.; Fraga, C.A.; et al. Design, synthesis and pharmacological profile of novel dopamine D2 receptor ligands. Bioorg. Med. Chem. 2003, 11, 4807–4813. [Google Scholar] [CrossRef]
- Neves, G.; Antonio, C.B.; Betti, A.H.; Pranke, M.A.; Fraga, C.A.M.; Barreiro, E.J.; Noël, F.; Rates, S.M.K. New insights into pharmacological profile of LASSBio-579, a multi-target N-phenylpiperazine derivative active on animal models of schizophrenia. Behav. Brain Res. 2013, 237, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Pompeu, T.E.T.; Alves, F.R.S.; Figueiredo, C.D.M.; Antonio, C.B.; Herzfeldt, V.; Moura, B.C.; Rates, S.M.K.; Barreiro, E.J.; Fraga, C.A.M.; Noël, F. Synthesis and pharmacological evaluation of new N-phenylpiperazine derivatives designed as homologues of the antipsychotic lead compound LASSBio-579. Eur. J. Med. Chem. 2013, 66, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Kaczor, A.A.; Targowska-Duda, K.M.; Budzyńska, B.; Biała, G.; Silva, A.G.; Castro, M. In vitro, molecular modeling and behavioral studies of 3-{[4-(5-methoxy-1H-indol-3-yl)-1,2,3,6-tetrahydropyridin-1-yl]methyl}-1,2-dihydroquinolin-2-one (D2AAK1) as a potential antipsychotic. Neurochem. Int. 2016, 96, 84–99. [Google Scholar] [CrossRef] [PubMed]
- Ivachtchenko, A.V.; Lavrovsky, Y.; Okun, I. AVN-101: A Multi-Target Drug Candidate for the Treatment of CNS Disorders. J. Alzheimers Dis. 2016, 53, 583–620. [Google Scholar] [CrossRef] [PubMed]
- Brea, J.; Castro, M.; Loza, M.I.; Masaguer, C.F.; Raviña, E.; Dezi, C.; Pastor, M.; Sanz, F.; Cabrero-Castel, A.; Galán-Rodríguez, B.; et al. QF2004B, a potential antipsychotic butyrophenone derivative with similar pharmacological properties to clozapine. Neuropharmacology 2006, 51, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhang, W.; Zhang, X.; Yin, L.; Chen, B.; Song, J. Synthesis and pharmacological evaluation of piperidine (piperazine)-substituted benzoxazole derivatives as multi-target antipsychotics. Bioorg. Med. Chem. Lett. 2015, 25, 5299–5305. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, S.; Xu, X.; Liu, X.; Yu, M.; Zhao, S.; Liu, S.; Qiu, Y.; Zhang, T.; Liu, B.-F.; et al. Synthesis and biological investigation of coumarin piperazine (piperidine) derivatives as potential multireceptor atypical antipsychotics. J. Med. Chem. 2013, 56, 4671–4690. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-W.; Sun, Y.-Y.; Fu, L.; Li, J.-Q. Synthesis and pharmacological characterization of novel N-(trans-4-(2-(4-(benzo[d]isothiazol-3-yl)piperazin-1-yl)ethyl)cyclohexyl)amides as potential multireceptor atypical antipsychotics. Eur. J. Med. Chem. 2016, 123, 332–353. [Google Scholar] [CrossRef] [PubMed]
- Xiamuxi, H.; Wang, Z.; Li, J.; Wang, Y.; Wu, C.; Yang, F.; Jiang, X.; Liu, Y.; Zhao, Q.; Chen, W.; et al. Synthesis and biological investigation of tetrahydropyridopyrimidinone derivatives as potential multireceptor atypical antipsychotics. Bioorg. Med. Chem. 2017, 25, 4904–4916. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Jiang, X.; Li, J.; Wang, Y.; Liu, Y.; Bi, M.; Wu, C.; Zhao, Q.; Chen, W.; Yin, J.; et al. Synthesis, structure-activity relationships, and biological evaluation of a series of benzamides as potential multireceptor antipsychotics. Bioorg. Med. Chem. Lett. 2016, 26, 3141–3147. [Google Scholar] [CrossRef] [PubMed]


















| Mechanism of Action | Clinical Efficacy | Possible Side Effects |
|---|---|---|
| D2 antagonism | ↓Positive symptoms | Extrapyramidal symptoms (EPS) ↓Negative symptoms ↑Cognitive symptoms ↑Drowsiness |
| D2 partial agonism | ↓Positive symptoms ↓Negative symptoms ↓Cognitive symptoms | Little or no EPS Behavioral activation |
| D3 antagonism | ↑Endocrine dysfunction ↑Weight gain ↑Sexual dysfunction | |
| 5-HT2A antagonism | ↓Negative symptoms | ↓EPS ↓Hyperprolactinemia |
| 5-HT1A partial agonism | ↓Negative symptoms ↓Cognitive symptoms ↓Anxiety symptoms ↓Depressive symptoms | ↓EPS ↓Hyperprolactinemia |
| 5-HT2C antagonism | ↑Weight gain ↑Appetite | |
| M1 antagonism | ↓EPS | ↑Anticholinergic symptoms, e.g., dry mouth, constipation, tachycardia ↑Drowsiness ↑Cognitive impairment |
| M1 agonism | ↓Psychotic symptoms ↓Cognitive symptoms | |
| M3 antagonism | ↑Type 2 diabetes mellitus ↑Hyperglycemic hyperosmolar syndrome ↑Diabetic ketoacidosis | |
| H1 antagonism | ↑Weight gain ↑Drowsiness ↑Hypotension | |
| α1-antagonism | ↑Dizziness ↑Drowsiness ↑Tachycardia ↓Blood pressure ↑Orthostatic hypotension | |
| α2-antagonism | ↓Depressive symptoms | ↑Anxiety ↑Tachycardia ↑Tremor ↑Dilated pupils ↑Sweating |
| β-antagonism | ↑Orthostatic hypotension ↑Sedation ↑Sexual dysfunction | |
| Glutamate modulation | ↓Positive symptoms ↓Negative symptoms ↓Cognitive symptoms ↓Illness progression |
| Drugs Generation | Examples | Receptors | Potential Side Effect | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| D1 | D2 | D3 | D4 | 5-HT2A | α1 | H1 | M1 | |||
| First | Chloropromazine | + | ++ | +++ | + | +++ | ++ | ++ | ++ | extrapyramidal symptoms such as dyskinesia, dystonias, akathisia, unwanted movements, muscle breakdown, tremors, rigidity and elevated prolactin |
| Haloperidol | + | +++ | + | + | 0 | + | 0 | 0 | ||
| Benperidol | 0 | +++ | ++ | ++ | + | 0 | 0 | |||
| Fluspirilene | + | +++ | +++ | + | 0 | 0 | 0 | |||
| Thioridazine | + | ++ | ++ | + | ++ | ++ | + | + | ||
| Second | Clozapine | ++ | + | ++ | +++ | ++ | + | +++ | hypotension, tachycardia, agranulocytosis | |
| Olanzapine | ++ | +++ | + | ++ | +++ | ++ | ++ | ++ | sedation, weight gain | |
| Risperidone | + | ++ | + | + | +++ | ++ | +++ | 0 | orthostatic hypotension, insomnia, restlessness, anxiety, headaches, agitation, extrapyramidal symptoms (EPS), rhinitis, sedation, fatigue, ocular disturbances, dizziness, palpitations, weight gain, diminished sexual desire, erectile and ejaculatory dysfunction, orthostatic dysregulation, reflex tachycardia, gastrointestinal complaints, nausea, rash, galactorrhea and amenorrhea | |
| Quetiapine | + | ++ | +++ | ++ | +++ | +++ | +++ | 0 | drowsiness, dizziness, headache, withdrawal symptoms, increased triglycerides, increased total cholesterol | |
| Ziprasidone | ++ | +++ | +++ | +++ | +++ | ++ | + | 0 | parkinsonism, headaches, rhinitis, orthostatic hypotension, tachykardia | |
| Third | Aripiprazole | 0 | +++ | ++ | + | +++ | + | + | 0 | hyperglycaemia, headache, extrapyramidal symptoms |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondej, M.; Stępnicki, P.; Kaczor, A.A. Multi-Target Approach for Drug Discovery against Schizophrenia. Int. J. Mol. Sci. 2018, 19, 3105. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19103105
Kondej M, Stępnicki P, Kaczor AA. Multi-Target Approach for Drug Discovery against Schizophrenia. International Journal of Molecular Sciences. 2018; 19(10):3105. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19103105
Chicago/Turabian StyleKondej, Magda, Piotr Stępnicki, and Agnieszka A. Kaczor. 2018. "Multi-Target Approach for Drug Discovery against Schizophrenia" International Journal of Molecular Sciences 19, no. 10: 3105. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19103105