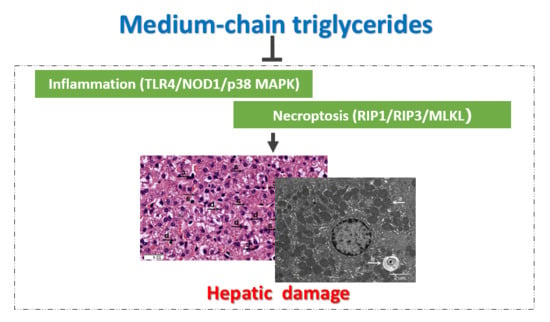
Medium-Chain Triglycerides Attenuate Liver Injury in Lipopolysaccharide-Challenged Pigs by Inhibiting Necroptotic and Inflammatory Signaling Pathways
Abstract
:1. Introduction
2. Results
2.1. MCT Supplementation Has No Effect on Growth Performance before LPS Challenge
2.2. MCT Supplementation Affects Liver Fatty Acid Composition after LPS Challenge
2.3. MCT Supplementation Attenuates Liver Morphological and Ultrastructural Destruction Challenged by LPS
2.4. MCT Supplementation Decreases Serum Alanine Aminotransferase (ALT) and Alkaline Phosphatase (AKP) activities after LPS Challenge
2.5. MCT Supplementation Increases Liver Claudin-1 and Heat Shock Protein 70 (HSP70) Protein Expression after LPS Challenge
2.6. MCT Supplementation Decrease Serum and Liver Proinflammatory Cytokine Concentrations and Inhibits Liver TLR4, NODs and Their Downstream Signals after LPS Challenge
2.7. MCT Supplementation Inhibits Liver mRNA or Protein Expression of Necroptosis-signaling Molecules after LPS Challenge
3. Discussion
4. Materials and Methods
4.1. Animal Care and Experimental Design
4.2. Blood and Liver Sample Collection
4.3. Assay of Liver Fatty Acid Composition
4.4. Liver Histological Examination
4.5. Ultrastructural Analysis of Hepatocytes
4.6. Assay of Serum Biochemical Parameters
4.7. Measurement of Serum and Liver Proinflammatory Cytokines
4.8. Western Blot Assay of Protein Expression
4.9. Real-time PCR Assay of mRNA Expression
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Racanelli, V.; Rehermann, B. The liver as an immunological organ. Hepatology 2006, 43, S54–S62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.; Liu, Y.; Zhu, H.; Hong, Y.; Wu, Z.; Hou, Y.; Li, Q.; Ding, B.; Yi, D.; Chen, H. Fish oil attenuates liver injury caused by LPS in weaned pigs associated with inhibition of TLR4 and nucleotide-binding oligomerization domain protein signaling pathways. Innate Immun. 2013, 19, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Thurman, R.G., II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am. J. Physiol. 1998, 275, G605–G611. [Google Scholar] [CrossRef] [PubMed]
- Diehl, A.M. Nonalcoholic steatosis and steatohepatitis IV. Nonalcoholic fatty liver disease abnormalities in macrophage function and cytokines. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 282, G1–G5. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.S.; Grøfte, T.; Tygstrup, N.; Vilstrup, H. Synthesis of acute phase proteins in rats with cirrhosis exposed to lipopolysaccharide. Comp. Hepatol. 2006, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Colletti, L.M.; Remick, D.G.; Burtch, G.D.; Kunkel, S.L.; Strieter, R.M.; Campbell, D.A., Jr. Role of tumor necrosis factor-alpha in the pathophysiologic alterations after hepatic ischemia/reperfusion injury in the rat. J. Clin. Invest. 1990, 85, 1936–1943. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, Y.; Che, Z.; Zhu, H.; Meng, G.; Hou, Y.; Ding, B.; Yin, Y.; Chen, F. Dietary L-arginine supplementation alleviates liver injury caused by Escherichia coli LPS in weaned pigs. Innate Immun. 2012, 18, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Zentek, J.; Buchheit-Renko, S.; Ferrara, F.; Vahjen, W.; Van Kessel, A.G.; Pieper, R. Nutritional and physiological role of medium-chain triglycerides and medium-chain fatty acids in piglets. Anim. Health Res. Rev. 2011, 12, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Łoś-Rycharska, E.; Kieraszewicz, Z.; Czerwionka-Szaflarska, M. Medium chain triglycerides (MCT) formulas in paediatric and allergological practice. Prz. Gastroenterol. 2016, 11, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Telliez, F.; Bach, V.; Leke, A.; Chardon, K.; Libert, J.P. Feeding behavior in neonates whose diet contained medium-chain triacylglycerols: Short-term effects on thermoregulation and sleep. Am. J. Clin. Nutr. 2002, 76, 1091–1095. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, N.; Suzuki, Y.; Nagatoishi, A.; Kasai, M.; Wu, J.; Taguchi, M. Effect of ingestion of medium-chain triacylglycerols on moderate- and high-intensity exercise in recreational athletes. J. Nutr. Sci. Vitaminol. (Tokyo) 2009, 55, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhong, W.; Qiu, Y.; Kang, X.; Sun, X.; Tan, X.; Zhao, Y.; Sun, X.; Jia, W.; Zhou, Z. Preservation of hepatocyte nuclear factor-4α contributes to the beneficial effect of dietary medium chain triglyceride on alcohol-induced hepatic lipid dyshomeostasis in rats. Alcohol. Clin. Exp. Res. 2013, 37, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Ronis, M.J.; Baumgardner, J.N.; Sharma, N.; Vantrease, J.; Ferguson, M.; Tong, Y.; Wu, X.; Cleves, M.A.; Badger, TM. Medium chain triglycerides dose-dependently prevent liver pathology in a rat model of non-alcoholic fatty liver disease. Exp. Biol. Med. 2013, 238, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.T. Review: Nutritional support for patients with cirrhosis. J. Gastroenterol. Hepatol. 1997, 12, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Afonso, M.B.; Rodrigues, P.M.; Simão, A.L.; Ofengeim, D.; Carvalho, T.; Amaral, J.D.; Gaspar, M.M.; Cortez-Pinto, H.; Castro, R.E.; Yuan, J.; et al. Activation of necroptosisin human and experimental cholestasis. Cell Death Dis. 2016, 7, e2390. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.M.; Kim, S.J.; Lee, S.M. Role of necroptosis in autophagy signaling during hepatic ischemia and reperfusion. Toxicol. Appl. Pharmacol. 2016, 308, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ni, H.M.; Dorko, K.; Kumer, S.C.; Schmitt, T.M.; Nawabi, A.; Komatsu, M.; Huang, H.; Ding, W.X. Increased hepatic receptor interacting protein kinase 3 expression due to impaired proteasomal functions contributes to alcohol-induced steatosis and liver injury. Oncotarget 2016, 7, 17681–17698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, D.; Prince, A. Participation of necroptosis in the host response to acute bacterial pneumonia. J. Innate Immun. 2017, 9, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; McQuade, T.; Siemer, A.B.; Napetschnig, J.; Moriwaki, K.; Hsiao, Y.S.; Damko, E.; Moquin, D.; Walz, T.; McDermott, A.; et al. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell 2012, 150, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Schmöcker, C.; Weylandt, K.H.; Kahlke, L.; Wang, J.; Lobeck, H.; Tiegs, G.; Berg, T.; Kang, J.X. Omega-3 fatty acids alleviate chemically induced acute hepatitis by suppression of cytokines. Hepatology 2007, 45, 864–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masaki, T.; Chiba, S.; Tatsukawa, H.; Yasuda, T.; Noguchi, H.; Seike, M.; Yoshimatsu, H. Adiponectin protects LPS-induced liver injury through modulation of TNF-alpha in KK-Ay obese mice. Hepatology 2004, 40, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.J.; Nandivada, P.; Chang, M.I.; Mitchell, P.D.; O’Loughlin, A.; Cowan, E.; Gura, K.M.; Nose, V.; Bistrian, B.R.; Puder, M. The addition of medium-chain triglycerides to a purified fish oil-based diet alters inflammatory profiles in mice. Metabolism 2015, 64, 274–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Chen, Y.; Li, Y.; Yang, L.; Wang, J.; Wang, T. Medium-chain TAG attenuate hepatic oxidative damage in intra-uterine growth-retarded weanling piglets by improving the metabolic efficiency of the glutathione redox cycle. Br. J. Nutr. 2014, 112, 876–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; Wang, Y.; Jiang, Y.; Zhang, Z.; Sun, X.; Yu, L.L. Dietary intake of structured lipids with different contents of medium-chain fatty acids on obesity prevention in C57BL/6J mice. J. Food Sci. 2017, 82, 1968–1977. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Fatty acids and inflammation: The cutting edge between food and pharma. Eur. J. Pharmacol. 2011, 668, S50–S58. [Google Scholar] [CrossRef] [PubMed]
- Giannini, E.G.; Testa, R.; Savarino, V. Liver enzyme alteration: A guide for clinicians. CMAJ 2005, 172, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Kono, H.; Fujii, H.; Asakawa, M.; Yamamoto, M.; Matsuda, M.; Maki, A.; Matsumoto, Y. Protective effects of medium-chain triglycerides on the liver and gut in rats administered endotoxin. Ann. Surg. 2003, 237, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.P.; Luk, J.M. Hepatic tight junctions: From viral entry to cancer metastasis. World J. Gastroenterol. 2010, 16, 289–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Chen, F.; Odle, J.; Lin, X.; Jacobi, S.K.; Zhu, H.; Wu, Z.; Hou, Y. Fish oil enhances intestinal integrity and inhibits TLR4 and NOD2 signaling pathways in weaned pigs after LPS challenge. J. Nutr. 2012, 142, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Kirpich, I.A.; Feng, W.; Wang, Y.; Liu, Y.; Barker, D.F.; Barve, S.S.; McClain, C.J. The type of dietary fat modulates intestinal tight junction integrity, gut permeability, and hepatic toll-like receptor expression in a mouse model of alcoholic liver disease. Alcohol. Clin. Exp. Res. 2012, 36, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Kanuri, G.; Spruss, A.; Wagnerberger, S.; Bischoff, S.C.; Bergheim, I. Role of tumor necrosis factor α (TNFα) in the onset of fructose-induced nonalcoholic fatty liver disease in mice. J. Nutr. Biochem. 2011, 22, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Dong, Z.; Wei, H. The involvement of heat shock proteins in murine liver regeneration. Cell Mol. Immunol. 2007, 4, 53–57. [Google Scholar] [PubMed]
- Oka, Y.; Akagi, Y.; Kinugasa, T.; Ishibashi, N.; Iwakuma, N.; Shiratsuchi, I.; Shirouzu, K. Heat-shock pre-treatment reduces liver injury and aids liver recovery after partial hepatectomy in mice. Anticancer Res. 2013, 33, 2887–2894. [Google Scholar] [PubMed]
- Nanji, A.A.; Jokelainen, K.; Tipoe, G.L.; Rahemtulla, A.; Dannenberg, A.J. Dietary saturated fatty acids reverse inflammatory and fibrotic changes in rat liver despite continued ethanol administration. J. Pharmacol. Exp. Ther. 2001, 299, 638–644. [Google Scholar] [PubMed]
- Geng, S.; Zhu, W.; Xie, C.; Li, X.; Wu, J.; Liang, Z.; Xie, W.; Zhu, J.; Huang, C.; Zhu, M.; et al. Medium-chain triglyceride ameliorates insulin resistance and inflammation in high fat diet-induced obese mice. Eur. J. Nutr. 2016, 55, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Rani, M.; Nicholson, S.E.; Zhang, Q.; Schwacha, M.G. Damage-associated molecular patterns (DAMPs) released after burn are associated with inflammation and monocyte activation. Burns 2017, 43, 297–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; Yuan, J. SnapShot: Necroptosis. Cell 2014, 158, 464. [Google Scholar] [CrossRef] [PubMed]
- Nikseresht, S.; Khodagholi, F.; Nategh, M.; Dargahi, L. RIP1 inhibition rescues from LPS-induced RIP3-mediated programmed cell death, distributed energy metabolism and spatial memory impairment. J. Mol. Neurosci. 2015, 57, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Kishida, E.; Tajiri, M.; Masuzawa, Y. Docosahexaenoic acid enrichment can reduce L929 cell necrosis induced by tumor necrosis factor. Biochim. Biophys. Acta 2006, 1761, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, F.J.; Almaguel, F.G.; Evans, W.; Rios-Colon, L.; Filippov, V.; Leoh, L.S.; Rook-Arena, E.; Mediavilla-Varela, M.; De Leon, M.; Casiano, C.A. Docosahexanoic acid antagonizes TNF-α-induced necroptosis by attenuating oxidative stress, ceramide production, lysosomal dysfunction, and autophagic features. Inflamm. Res. 2014, 63, 859–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NRC. Nutrient Requirements of Swine; National Academies Press: Washington, DC, USA, 1998. [Google Scholar]
- Nieto, N.; Torres, M.I.; Ríos, A.; Gil, A. Dietary polyunsaturated fatty acids improve histological and biochemical alterations in rats with experimental ulcerative colitis. J. Nutr. 2002, 132, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]






| Item | Saline | LPS | SEM | P Value | ||||
|---|---|---|---|---|---|---|---|---|
| Control | MCTs | Control | MCTs | Diet | LPS | Interaction | ||
| 2 h | ||||||||
| ALT, U/L | 73.2 | 60.1 | 73.1 | 50.1 | 8.0 | 0.035 | 0.53 | 0.54 |
| AST, U/L | 97 | 92 | 97 | 88 | 15 | 0.64 | 0.88 | 0.90 |
| ALT/AST | 0.783 | 0.815 | 0.775 | 0.633 | 0.128 | 0.67 | 0.47 | 0.50 |
| AKP, U/L | 279 | 233 | 319 | 279 | 24 | 0.09 | 0.09 | 0.90 |
| GGT, U/L | 37.3 | 44.0 | 45.2 | 57.0 | 6.1 | 0.14 | 0.09 | 0.69 |
| 4 h | ||||||||
| ALT, U/L | 75.7 | 62.4 | 71.7 | 52.7 | 7.0 | 0.033 | 0.34 | 0.69 |
| AST, U/L | 85 | 92 | 159 | 160 | 25 | 0.88 | 0.010 | 0.91 |
| ALT/AST | 0.895 | 0.850 | 0.543 | 0.435 | 0.131 | 0.57 | 0.009 | 0.81 |
| AKP, U/L | 280 | 242 | 422 | 304 | 29 | 0.013 | 0.002 | 0.18 |
| GGT, U/L | 34.5 | 43.2 | 63.2 | 74.0 | 7.3 | 0.20 | 0.001 | 0.89 |
| Item | Saline | LPS | SEM | P Value | ||||
|---|---|---|---|---|---|---|---|---|
| Control | MCTs | Control | MCTs | Diet | LPS | Interaction | ||
| TLR4 | 1.00 ab | 0.92 a | 1.70 b | 0.71 a | 0.24 | 0.035 | 0.31 | 0.07 |
| MyD88 | 1.00 | 0.84 | 1.93 | 1.16 | 0.18 | 0.020 | 0.003 | 0.11 |
| IRAK1 | 1.00 ab | 0.87 a | 1.47 b | 0.70 a | 0.16 | 0.012 | 0.37 | 0.06 |
| TRAF6 | 1.00 | 0.80 | 1.15 | 0.69 | 0.14 | 0.032 | 0.90 | 0.38 |
| NOD1 | 1.00 | 0.77 | 1.78 | 0.84 | 0.21 | 0.013 | 0.06 | 0.11 |
| NOD2 | 1.00 | 1.41 | 7.03 | 6.19 | 0.60 | 0.72 | <0.001 | 0.31 |
| RIP2 | 1.00 a | 0.77 a | 7.55c | 3.87 b | 0.70 | 0.011 | <0.001 | 0.023 |
| NF-κB | 1.00 | 0.77 | 1.40 | 0.88 | 0.17 | 0.045 | 0.16 | 0.42 |
| TNF-α | 1.00 a | 1.43 ab | 4.25 c | 2.28 b | 0.35 | 0.040 | <0.001 | 0.003 |
| IL-1β | 1.00 a | 1.04 a | 47.38 c | 20.36 b | 5.74 | 0.029 | <0.001 | 0.029 |
| IL-6 | 1.00 | 1.16 | 14.13 | 14.20 | 2.41 | 0.96 | <0.001 | 0.98 |
| Item | Saline | LPS | SEM | P Value | ||||
|---|---|---|---|---|---|---|---|---|
| Control | MCTs | Control | MCTs | Diet | LPS | Interaction | ||
| RIP1 | 1.00 | 0.79 | 2.77 | 2.74 | 0.24 | 0.62 | < 0.001 | 0.72 |
| RIP3 | 1.00 | 0.72 | 0.81 | 0.38 | 0.12 | 0.007 | 0.040 | 0.56 |
| MLKL | 1.00 a | 0.94 a | 12.23 c | 5.36 b | 1.17 | 0.008 | < 0.001 | 0.009 |
| FADD | 1.00 a | 1.63 b | 1.54 ab | 1.12 ab | 0.18 | 0.57 | 0.93 | 0.009 |
| PGAM5 | 1.00 a | 0.89 a | 2.79 c | 1.82 b | 0.24 | 0.037 | <0.001 | 0.09 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Wang, X.; Chen, S.; Wang, S.; Tu, Z.; Zhang, G.; Zhu, H.; Li, X.; Xiong, J.; Liu, Y. Medium-Chain Triglycerides Attenuate Liver Injury in Lipopolysaccharide-Challenged Pigs by Inhibiting Necroptotic and Inflammatory Signaling Pathways. Int. J. Mol. Sci. 2018, 19, 3697. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19113697
Zhang L, Wang X, Chen S, Wang S, Tu Z, Zhang G, Zhu H, Li X, Xiong J, Liu Y. Medium-Chain Triglycerides Attenuate Liver Injury in Lipopolysaccharide-Challenged Pigs by Inhibiting Necroptotic and Inflammatory Signaling Pathways. International Journal of Molecular Sciences. 2018; 19(11):3697. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19113697
Chicago/Turabian StyleZhang, Lin, Xiuying Wang, Shaokui Chen, Shuhui Wang, Zhixiao Tu, Guolong Zhang, Huiling Zhu, Xiangen Li, Jianglin Xiong, and Yulan Liu. 2018. "Medium-Chain Triglycerides Attenuate Liver Injury in Lipopolysaccharide-Challenged Pigs by Inhibiting Necroptotic and Inflammatory Signaling Pathways" International Journal of Molecular Sciences 19, no. 11: 3697. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19113697