Melanogenesis Inhibitors from the Rhizoma of Ligusticum Sinense in B16-F10 Melanoma Cells In Vitro and Zebrafish In Vivo
Abstract
:1. Introduction
2. Results and Discussions
2.1. Isolation and Structural Elucidation
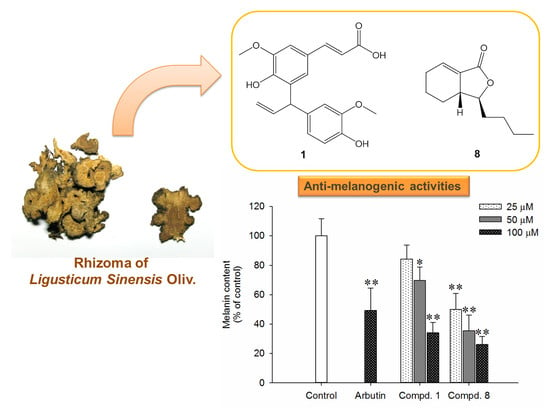
2.2. Effects of Compounds 1 and 8 on Melanin Content in α-MSH-Stimulated B16-F10 Cells
2.3. In Vivo Zebrafish Pigmentation Assay
2.4. Viability Assay of Human Epidermal Skin Equivalents
2.5. Molecular Docking Study of B16-Mus Musculus Tyrosinase
3. Materials and Methods
3.1. General
3.2. Plant Materials
3.3. Isolation and Structural Elucidation
3.4. Spectroscopic Data
3.5. HPLC–DAD Analysis
3.6. Cell Culture
3.7. Cell Viability Assay
3.8. Melanin Content Assay
3.9. In Vivo Zebrafish Pigmentation Assay
3.10. Viability Assay on Normal Human Epidermal Keratinocytes
3.11. Statistical Analysis
3.12. Molecular Docking Study of B16-Mus Musculus Tyrosinase
3.12.1. Homology Modeling
3.12.2. Analysis of Ligand-Protein Interaction
4. Conclusions
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin Pigmentation in Mammalian Skin and Its Hormonal Regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, I.; Hong, S. Neural Stem Cells and Its Derivatives as a New Material for Melanin Inhibition. Int. J. Mol. Sci. 2018, 19, 36. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, S.A.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling Pathways in Melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.A.; Fisher, D.E. The melanoma revolution: From UV carcinogenesis to a new era in therapeutics. Science 2014, 346, 945–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Weng, Q.Y.; Fisher, D.E. UV signaling pathways within the skin. J. Investig. Dermatol. 2014, 134, 2080–2085. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Jung, S.H. Recent development of signaling pathways inhibitors of melanogenesis. Cell Signal. 2017, 40, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Couteau, C.; Coiffard, L. Overview of Skin Whitening Agents: Drugs and Cosmetic Products. Cosmetics 2016, 3, 27. [Google Scholar] [CrossRef]
- Zhu, W.; Gao, J. The use of botanical extracts as topical skin-lightening agents for the improvement of skin pigmentation disorders. J. Investig. Dermatol. Symp. Proc. 2008, 13, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.-Y. Food Plants of China; Chinese University Press: Hong Kong, China, 2005. [Google Scholar]
- Chen, D.W. Shen Nong’s Herbal Classic Collections, 1st ed.; Tianjin Science and Technology Press: Tianjin, China, 2010. [Google Scholar]
- Ma, J.-P.; Tan, C.-H.; Zhu, D.-Y. Chemical Constituents of Ligusticum sinensis Oliv. Helv. Chim. Acta 2007, 90, 158–163. [Google Scholar] [CrossRef]
- Yu, D.Q.; Chen, R.Y.; Xie, F.Z. Structure elucidation of ligustilone from Ligusticum sinensis Oliv. Chin. Chem. Lett. 1995, 6, 391–394. [Google Scholar]
- Yu, D.Q.; Xie, F.Z.; Chen, R.Y.; Huang, Y.H. Studies on the structure of ligustiphenol from Ligusticum sinense Oliv. Chin. Chem. Lett. 1996, 7, 721–722. [Google Scholar]
- Zhang, Y.-C.; Chen, C.; Li, S.-J.; Xu, H.-Y.; Li, D.-F.; Wu, H.-W.; Yang, H.-J. Chemical analysis and observation on vascular activity of essential oil from Ligusticum sinensis, Conioselinum tataricum and Ligusticum jeholense. Chin. J. Exp. Tradit. Med. Formulae 2011, 17, 159–165. [Google Scholar] [CrossRef]
- Wang, J.; Xu, L.; Yang, L.; Liu, Z.; Zhou, L. Composition, antibacterial and antioxidant activities of essential oils from Ligusticum sinense and L. jeholense (Umbelliferae) from China. Rec. Nat. Prod. 2011, 5, 314–318. [Google Scholar]
- Wang, J.; Yang, J.-B.; Wang, A.-G.; Ji, T.-F.; Su, Y.-L. Studies on the chemical constituents of Ligusticum sinense. Zhong Yao Cai 2011, 34, 378–380. [Google Scholar] [PubMed]
- Zhang, J.; Zhou, Z.; Chen, R.; Xie, F.; Cheng, G.; Yu, D.; Zhou, T. Study on chemistry and pharmacology of genus Ligusticum. Chin. Pharm. J. 2002, 37, 654–657. [Google Scholar] [CrossRef]
- Du, J.; Yu, Y.; Ke, Y.; Wang, C.; Zhu, L.; Qian, Z.M. Ligustilide attenuates pain behavior induced by acetic acid or formalin. J. Ethnopharmacol. 2007, 112, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Shin, J.S.; Jang, D.S.; Lee, K.T. Cnidilide, an alkylphthalide isolated from the roots of Cnidium officinale, suppresses LPS-induced NO, PGE2, IL-1beta, IL-6 and TNF-alpha production by AP-1 and NF-kappaB inactivation in RAW 264.7 macrophages. Int. Immunopharmacol. 2016, 40, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, Y.; Sekita, S.; Harada, M. Centrally acting muscle relaxant effect of phthalides (ligustilide, cnidilide and senkyunolide) obtained from Cnidium officinale Makino. Yakugaku Zasshi 1989, 109, 402–406. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Pang, J.-H.S.; Huang, S.-T. Inhibition of Melanogenesis in Murine B16/F10 Melanoma Cells by Ligusticum sinensis Oliv. Am. J. Chin. Med. 2006, 34, 523–533. [Google Scholar] [CrossRef]
- Chu, Y.T. Studies on Constituents and Melanogenesis-Inhibitory Effects of Ligusticum sinense; Taipei Medical University: Taipei, Taiwan, 2011. [Google Scholar]
- Niu, C.; Yin, L.; Aisa, H.A. Novel Furocoumarin Derivatives Stimulate Melanogenesis in B16 Melanoma Cells by Up-Regulation of MITF and TYR Family via Akt/GSK3beta/beta-Catenin Signaling Pathways. Int. J. Mol. Sci. 2018, 19, 746. [Google Scholar] [CrossRef]
- Chan, Y.Y.; Kim, K.H.; Cheah, S.H. Inhibitory effects of Sargassum polycystum on tyrosinase activity and melanin formation in B16F10 murine melanoma cells. J. Ethnopharmacol. 2011, 137, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Kim, J.; Jang, J.H.; Lee, S.; Park, C.M.; Kim, W.K.; Kim, J.S. Novel (1E,3E,5E)-1,6-bis(Substituted phenyl)hexa-1,3,5-triene Analogs Inhibit Melanogenesis in B16F10 Cells and Zebrafish. Int. J. Mol. Sci. 2018, 19, 1067. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Hisama, M. Suppression of Chemical Mutagen-Induced SOS Response by Alkylphenols from Clove (Syzygium aromaticum) in the Salmonella typhimurium TA1535/pSK1002 umu Test. J. Agric. Food Chem. 2001, 49, 4019–4025. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Z.-I.; Haruta, M. Effects of substituents on intramolecular hydrogen bonds of 2-hydroxy-4-substituted acetophenones. Tetrahedron Lett. 1965, 6, 3745–3751. [Google Scholar] [CrossRef]
- Bartschat, D.; Beck, T.; Mosandl, A. Stereoisomeric Flavor Compounds. 79. Simultaneous Enantio-selective Analysis of 3-Butylphthalide and 3-Butylhexahydro-phthalide Stereoisomers in Celery, Celeriac, and Fennel. J. Agric. Food Chem. 1997, 45, 4554–4557. [Google Scholar] [CrossRef]
- Yamada, K.; Murata, T.; Kobayashi, K.; Miyase, T.; Yoshizaki, F. A lipase inhibitor monoterpene and monoterpene glycosides from Monarda punctata. Phytochemistry 2010, 71, 1884–1891. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Moriyasu, M.; Ichimaru, M.; Tachibana, Y.; Kato, A.; Mathenge, S.G.; Nganga, J.N.; Juma, F.D. Acyclic triterpenoids from Ekebergia capensis. Phytochemistry 1996, 42, 803–807. [Google Scholar] [CrossRef]
- Oguro, D.; Watanabe, H. Synthesis and sensory evaluation of all stereoisomers of sedanolide. Tetrahedron 2011, 67, 777–781. [Google Scholar] [CrossRef]
- Naito, T.; Niitsu, K.; Ikeya, Y.; Okada, M.; Mitsuhashi, H. A phthalide and 2-farnesyl-6-methyl benzoquinone from Ligusticum chuangxiong. Phytochemistry 1992, 31, 1787–1789. [Google Scholar] [CrossRef]
- Kong, C.-S.; Um, Y.-R.; Lee, J.-I.; Kim, Y.-A.; Yea, S.-S.; Seo, Y. Constituents isolated from Glehnia littoralis suppress proliferations of human cancer cells and MMP expression in HT1080 cells. Food Chem. 2010, 120, 385–394. [Google Scholar] [CrossRef]
- Miyazawa, M.; Tsukamoto, T.; Anzai, J.; Ishikawa, Y. Insecticidal Effect of Phthalides and Furanocoumarins from Angelica acutiloba against Drosophila melanogaster. J. Agric. Food Chem. 2004, 52, 4401–4405. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.J.; Xu, G.H.; Sun, X.; Peng, Y.; Ji, X.; Jiang, K.; Li, F. Synthesis of Bosutinib from 3-Methoxy-4-hydroxybenzoic Acid. Molecules 2010, 15, 4261–4266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.-T.; Fu, X.-L.; Tan, C.; Zeng, Y.; Wang, Q.; Zhao, P.-J. Two new chroman derivations from the endophytic Penicillium sp. DCS523. Molecules 2011, 16, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Ritter, T.; Stanek, K.; Larrosa, I.; Carreira, E.M. Mild Cleavage of Aryl Mesylates: Methanesulfonate as Potent Protecting Group for Phenols. Org. Lett. 2004, 6, 1513–1514. [Google Scholar] [CrossRef] [PubMed]
- Faustino, H.; Gil, N.; Baptista, C.; Duarte, A.P. Antioxidant activity of lignin phenolic compounds extracted from kraft and sulphite black liquors. Molecules 2010, 15, 9308–9322. [Google Scholar] [CrossRef] [PubMed]
- Tamura, S.; Ohno, T.; Hattori, Y.; Murakami, N. Establishment of absolute stereostructure of falcarindiol, algicidal principle against Heterocapsa circularisquama from Notopterygii Rhizoma. Tetrahedron Lett. 2010, 51, 1523–1525. [Google Scholar] [CrossRef]
- Ohta, T.; Uwai, K.; Kikuchi, R.; Nozoe, S.; Oshima, Y.; Sasaki, K.; Yoshizaki, F. Absolute stereochemistry of cicutoxin and related toxic polyacetylenic alcohols from Cicuta virosa. Tetrahedron 1999, 55, 12087–12098. [Google Scholar] [CrossRef]
- Bibi, N.; Tanoli, S.A.K.; Farheen, S.; Afza, N.; Siddiqi, S.; Zhang, Y.; Kazmi, S.U.; Malik, A. In vitro antituberculosis activities of the constituents isolated from Haloxylon salicornicum. Bioorg. Med. Chem. Lett. 2010, 20, 4173–4176. [Google Scholar] [CrossRef] [PubMed]
- Shingate, B.B.; Hazra, B.G.; Pore, V.S.; Gonnade, R.G.; Bhadbhade, M.M. Stereoselective syntheses of 20-epi cholanic acid derivatives from 16-dehydropregnenolone acetate. Tetrahedron 2007, 63, 5622–5635. [Google Scholar] [CrossRef]
- Cantrell, C.L.; Case, B.P.; Mena, E.E.; Kniffin, T.M.; Duke, S.O.; Wedge, D.E. Isolation and Identification of Antifungal Fatty Acids from the Basidiomycete Gomphus floccosus. J. Agric. Food Chem. 2008, 56, 5062–5068. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Parkin, K. Isolation and Identification of Phase II Enzyme-Inducing Agents from Nonpolar Extracts of Green Onion (Allium spp.). J. Agric. Food Chem. 2006, 54, 8417–8424. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, Q.-W.; Li, S.-P. Preparative purification of coniferyl ferulate from Angelica sinensis oil by high performance centrifugal partition chromatography. J. Med. Plants Res. 2011, 5, 104–108. [Google Scholar]
- Gopalakrishnan, S.; Subbarao, G.V.; Nakahara, K.; Yoshihashi, T.; Ito, O.; Maeda, I.; Ono, H.; Yoshida, M. Nitrification Inhibitors from the Root Tissues of Brachiaria humidicola, a Tropical Grass. J. Agric. Food Chem. 2007, 55, 1385–1388. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-K.; Lu, C.-K.; Kuo, Y.-H.; Chen, J.-Z.; Sun, G.-Z. New Prenylated Flavones from the Roots of Ficus Beecheyana. J. Chin. Chem. Soc. 2004, 51, 437–441. [Google Scholar] [CrossRef]
- Yao, C.; Oh, J.H.; Oh, I.G.; Park, C.H.; Chung, J.H. [6]-Shogaol inhibits melanogenesis in B16 mouse melanoma cells through activation of the ERK pathway. Acta Pharmacol. Sin. 2013, 34, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A. Subchapter 16B—Melanocyte-Stimulating Hormone. In Handbook of Hormones; Takei, Y., Ando, H., Tsutsui, K., Eds.; Academic Press: San Diego, CA, USA, 2016; p. 120-e16B-7. [Google Scholar]
- Choi, T.Y.; Kim, J.H.; Ko, D.H.; Kim, C.H.; Hwang, J.S.; Ahn, S.; Kim, S.Y.; Kim, C.D.; Lee, J.H.; Yoon, T.J. Zebrafish as a new model for phenotype-based screening of melanogenic regulatory compounds. Pigment Cell Res. 2007, 20, 120–127. [Google Scholar] [CrossRef]
- Nakagawa, M.; Kawai, K.; Kawai, K. Contact allergy to kojic acid in skin care products. Contact Dermatitis 1995, 32, 9–13. [Google Scholar] [CrossRef]
- Nohynek, G.J.; Kirkland, D.; Marzin, D.; Toutain, H.; Leclerc-Ribaud, C.; Jinnai, H. An assessment of the genotoxicity and human health risk of topical use of kojic acid [5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one]. Food Chem. Toxicol. 2004, 42, 93–105. [Google Scholar] [CrossRef]
- El Ghalbzouri, A.; Commandeur, S.; Rietveld, M.H.; Mulder, A.A.; Willemze, R. Replacement of animal-derived collagen matrix by human fibroblast-derived dermal matrix for human skin equivalent products. Biomaterials 2009, 30, 71–78. [Google Scholar] [CrossRef]
- Solano, F.; Briganti, S.; Picardo, M.; Ghanem, G. Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006, 19, 550–571. [Google Scholar] [CrossRef]
- Smit, N.; Vicanova, J.; Pavel, S. The hunt for natural skin whitening agents. Int. J. Mol. Sci. 2009, 10, 5326–5349. [Google Scholar] [CrossRef] [PubMed]
- Tiechi, L.; Wenyuan, Z.; Mingyu, X. Studies on the Effect of TCM on Melanin Biosynthesis I. Inhibitory Actions of Ethanolic Extracts of 82 Different Chinese Crude Drugs on Tyrosinase Activity. Chin. Tradit. Herb. Drugs 1999, 30, 336–339. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Siegrist, W.; Eberle, A.N. In situ melanin assay for MSH using mouse B16 melanoma cells in culture. Anal. Biochem. 1986, 159, 191–197. [Google Scholar] [CrossRef]
- Commandeur, S.; De Gruijl, F.R.; Willemze, R.; Tensen, C.P.; El Ghalbzouri, A. An in vitro three-dimensional model of primary human cutaneous squamous cell carcinoma. Exp. Dermatol. 2009, 18, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Position | 13C NMR a | 1H NMR | HMBC |
|---|---|---|---|
| δC (multi.) | δH (multi., J in Hz) | H→C | |
| 1 | 126.7 (s) | ||
| 2 | 108.9 (d) | 7.22 (d, 1.8) | C-1, C-3, C-4, C-6 |
| 3 | 148.6 (s) | ||
| 4 | 147.2 (s) | ||
| 5 | 131.2 (s) | ||
| 6 | 123.8 (d) | 7.07 (d, 1.8) | C-2, C-4, C-7′ |
| 7 | 146.2 (d) | 7.56 (d, 15.9) | C-1, C-2, C-6, C-8, C-9 |
| 8 | 116.1 (d) | 6.33 (d, 15.9) | C-1, C-7, C-9 |
| 9 | 168.4 (s) | ||
| 1′ | 135.3 (s) | ||
| 2′ | 113.2 (d) | 6.87 (d, 1.8) | C-1′, C-3′, C-4′, C-6′, C-7′ |
| 3′ | 148.2 (s) | ||
| 4′ | 146.1 (s) | ||
| 5′ | 115.6 (d) | 6.74 (d, 7.9) | C-1′, C-3′, C-4′ |
| 6′ | 121.8 (d) | 6.69 (dd, 7.9, 1.8) | C-2′, C-4′, C-7′ |
| 7′ | 48.2 (d) | 5.10 (br d, 7.6) | C-4, C-5, C-6, C-1′, C-2′, C-6′, C-8′, C-9′ |
| 8′ | 141.5 (d) | 6.40 (ddd, 17.1, 10.1, 7.6) | C-5, C-1′, C-7′ |
| 9′ | 115.9 (t) | 4.99 (ddd, 17.1, 1.8, 1.8) | C-7′, C-8′ |
| 5.15 (ddd, 10.1, 1.8, 1.8) | C-7′ | ||
| 3-OCH3 | 56.6 (q) | 3.92 (3H, s) | C-3 |
| 3′-OCH3 | 56.4 (q) | 3.77 (3H, s) | C-3′ |
| Position | 13C NMR a | 1H NMR | HMBC |
|---|---|---|---|
| δC (multi.) | δH (multi., J in Hz) | H→C | |
| 1 | 69.2 (d) | 3.73 (dt, 9.1, 4.2) | C-3, C-5 |
| 2 | 67.1 (d) | 4.08 (br t, 4.2) | C-1, C-3, C-4, C-6 |
| 3 | 121.0 (d) | 5.44 (m) | C-1, C-2, C-5, C-7 |
| 4 | 144.1 (s) | ||
| 5 | 27.2 (t) | 1.92–2.01 (m) | C-1 |
| 2.04–2.10 (m) | C-1, C-3, C-4, C-6, C-7 | ||
| 6 | 26.3 (t) | 1.66–1.78 (m) | C-1, C-2, C-5 |
| 7 | 37.4 (t) | 1.97 (br t, 7.3) | C-4, C-9 |
| 8 | 27.4 (t) | 1.38 (m) | C-4, C-7, C-9, C-10 |
| 9 | 31.8 (t) | 1.20 (m) | C-7, C-10 |
| 10 | 22.7 (t) | 1.27 (m) | C-8, C-9, C-11 |
| 11 | 14.2 (q) | 0.85 (t, 7.0) | C-9, C-10 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, M.-C.; Lee, T.-H.; Chu, Y.-T.; Syu, L.-L.; Hsu, S.-J.; Cheng, C.-H.; Wu, J.; Lee, C.-K. Melanogenesis Inhibitors from the Rhizoma of Ligusticum Sinense in B16-F10 Melanoma Cells In Vitro and Zebrafish In Vivo. Int. J. Mol. Sci. 2018, 19, 3994. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19123994
Cheng M-C, Lee T-H, Chu Y-T, Syu L-L, Hsu S-J, Cheng C-H, Wu J, Lee C-K. Melanogenesis Inhibitors from the Rhizoma of Ligusticum Sinense in B16-F10 Melanoma Cells In Vitro and Zebrafish In Vivo. International Journal of Molecular Sciences. 2018; 19(12):3994. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19123994
Chicago/Turabian StyleCheng, Min-Chi, Tzong-Huei Lee, Yi-Tzu Chu, Li-Ling Syu, Su-Jung Hsu, Chia-Hsiung Cheng, Jender Wu, and Ching-Kuo Lee. 2018. "Melanogenesis Inhibitors from the Rhizoma of Ligusticum Sinense in B16-F10 Melanoma Cells In Vitro and Zebrafish In Vivo" International Journal of Molecular Sciences 19, no. 12: 3994. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19123994