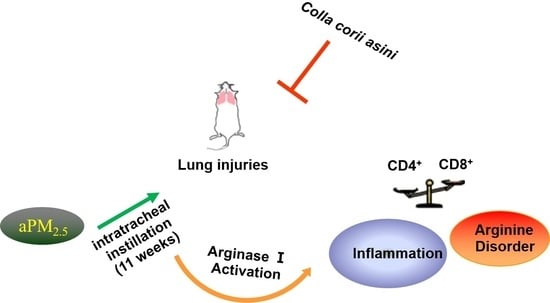
Protective Effect of Colla corii asini against Lung Injuries Induced by Intratracheal Instillation of Artificial Fine Particles in Rats
Abstract
:1. Introduction
2. Results
2.1. Colla corii asini Protects Rats from aPM2.5-Induced Lung Function Changes
2.2. Effect of Colla corii asini on aPM2.5-Induced Alteration of T Lymphocyte Subsets
2.3. Effect of Colla corii asini on Cell Counts and Percentages
2.4. Effect of Colla corii asini on Protein and mRNA Expression of TNF-α, IL-1β and IL-10
2.5. Colla corii asini Inhibits Pathologic Changes and Lung Injury in Rats
2.6. Role of Colla corii asini in Protection against aPM2.5-Induced Alteration of Amino Metabolic Profile
3. Discussion
4. Materials and Methods
4.1. Chemical Reagents
4.2. Animal Handling
4.3. Rat model of Intratracheal Instillation of aPM2.5 and the Administration of Colla corii asini
4.4. Pulmonary Function Measurement
4.5. T Lymphocyte Phenotype Analysis of Whole Blood
4.6. Bronchoalveolar Lavage Fluid Collection and Analysis
4.7. Lung Histopathology Analysis
4.8. LC-MS/MS-Based Metabolomics Analysis
4.9. Quantitative Reverse-Transcriptase DNA Polymerase Chain Reaction (qRT-PCR) Analysis
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| aPM | Artificial particular matter |
| ACTB | β-actin |
| BALF | Bronchoalveolar lavage fluid |
| LC-MS/MS | Liquid chromatograph-mass spectrometer/ mass spectrometer |
| IL | Interleukin |
References
- Di, Q.; Wang, Y.; Zanobetti, A.; Wang, Y.; Koutrakis, P.; Choirat, C.; Dominici, F.; Schwartz, J.D. Air Pollution and Mortality in the Medicare Population. N. Engl. J. Med. 2017, 376, 2513–2522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landrigan, P.J.; Fuller, R.; Acosta, N.J.R.; Adeyi, O.; Arnold, R.; Basu, N.; Baldé, A.B.; Bertollini, R.; Bose-O’Reilly, S.; Boufford, J.I.; et al. The Lancet Commission on pollution and health. Lancet 2018, 391, 462–512. [Google Scholar] [CrossRef]
- Landrigan, P.J. Air pollution and health. Lancet Public Health 2017, 2, e4–e5. [Google Scholar] [CrossRef]
- Guan, W.-J.; Zheng, X.-Y.; Chung, K.F.; Zhong, N.-S. Impact of air pollution on the burden of chronic respiratory diseases in China: Time for urgent action. Lancet 2016, 388, 1939–1951. [Google Scholar] [CrossRef]
- Yin, P.; He, G.; Fan, M.; Chiu, K.Y.; Fan, M.; Liu, C.; Xue, A.; Liu, T.; Pan, Y.; Mu, Q. Particulate air pollution and mortality in 38 of China’s largest cities: Time series analysis. BMJ 2017, 356, j667. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, J.; Yang, L.; Xu, Y.; Zhang, X.; Bai, C.; Kang, J.; Ran, P.; Shen, H.; Wen, F. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): A national cross-sectional study. Lancet 2018, 391, 1706–1717. [Google Scholar] [CrossRef]
- Ni, L.; Chuang, C.-C.; Zuo, L. Fine particulate matter in acute exacerbation of COPD. Front. Physiol. 2015, 6, 294. [Google Scholar] [CrossRef] [PubMed]
- Cosselman, K.E.; Navas-Acien, A.; Kaufman, J.D. Environmental factors in cardiovascular disease. Nat. Rev. Cardiol. 2015, 12, 627. [Google Scholar] [CrossRef]
- Nel, A. Air pollution-related illness: Effects of particles. Science 2005, 308, 804–806. [Google Scholar] [CrossRef]
- Wang, H.; Naghavi, M.; Allen, C.; Barber, R.M.; Bhutta, Z.A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; Coates, M.M. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef]
- Liu, M.; Tan, H.; Zhang, X.; Liu, Z.; Cheng, Y.; Wang, D.; Wang, F. Hematopoietic effects and mechanisms of Fufang ejiao jiang on radiotherapy and chemotherapy-induced myelosuppressed mice. J. Ethnopharmacol. 2014, 152, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; You, J.; Tian, S.; Zhang, Y.; Feng, M. Overview of pharmacological and clinical study on compound Ejiao Jiang. China J. Chin. Mater. Med. 2012, 37, 3021–3023. [Google Scholar]
- Wu, H.; Yang, F.; Cui, S.; Qin, Y.; Liu, J.; Zhang, Y. Hematopoietic effect of fractions from the enzyme-digested colla corii asini on mice with 5-fluorouracil induced anemia. Am. J. Chin. Med. 2007, 35, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, M.; Cao, J.; Cheng, Y.; Zhuo, C.; Xu, H.; Tian, S.; Zhang, Y.; Zhang, J.; Wang, F. Effect of Colla corii asini (E’jiao) on D-galactose induced aging mice. Biol. Pharm. Bull. 2012, 35, 2128–2132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, F.; Li, B.; Yang, Y.; Tian, S.; Li, Z. Effect of colla corri asini on immune function in mice. Sci. Technol. Food Ind. 2011, 32, 400–402. [Google Scholar]
- Zhang, Z.; Ma, Y.; Hu, J.; Lan, H.; Zhang, Y.; Yao, C. Reverse effect of Ejiao (colla corri asini) on imbalance of Th17/Treg subsets of mice with airway inflammation. Shandong Med. J. 2018, 6, 11–14. [Google Scholar]
- Piaopiao, Z.; Yahao, L.; Xiaodan, Y.; Jinfeng, W.; Hongtao, J.; Aiping, W. Protective effects of Colla corii asini in on artificial fine particulate matter-induced respiratory system injury in rats. Carcinog. Teratog. Mutagen. 2017, 29, 346–351. [Google Scholar]
- Zhang, W.; Sun, Y.; Zhuang, G.; Xu, D. Characteristics and seasonal variations of PM2.5, PM10, and TSP aerosol in Beijing. Biomed. Environ. Sci. 2006, 19, 461. [Google Scholar]
- Jerrett, M. Atmospheric science: The death toll from air-pollution sources. Nature 2015, 525, 330. [Google Scholar] [CrossRef]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef]
- Raaschou-Nielsen, O.; Andersen, Z.J.; Beelen, R.; Samoli, E.; Stafoggia, M.; Weinmayr, G.; Hoffmann, B.; Fischer, P.; Nieuwenhuijsen, M.J.; Brunekreef, B. Air pollution and lung cancer incidence in 17 European cohorts: Prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). Lancet Oncol. 2013, 14, 813–822. [Google Scholar] [CrossRef]
- Yan, X.D.; Wang, Q.M.; Cai, T.; Jin, H.T.; Han, Y.X.; Zhang, J.L.; Yu, X.M.; Hou, Q.; Zhang, P.P.; Wang, A.P. Polydatin protects the respiratory system from PM2.5 exposure. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Dong, J.; Cui, Y.; Xie, J.; Wu, S. The effect of Ejiao on airway inflammation and Th1/Th2 cytokines in serum of asthmatic rats. Chin. J. Exp. Tradit. Med. Form. 2006, 12, 59–61. [Google Scholar]
- Zhang, Z.; Li, N.; Liu, Q.; Ma, Y.; Zhang, Y.; Lan, H.-Y.; Yao, C.-F. Protective Effect of Ejiao on COPD Model Mice and the Effect on MMP-2,MMP-9 and TGF-β1 Levels. Genom. Appl. Biol. 2018, 37, 1813–1819. [Google Scholar]
- De Fcajfc, A.J.; Der Plaat, D.A.; de Jong, K.; van Diemen, C.; Dirkje, S.; Nedeljkovic, I.; van Duijn, C.; Amin, N.; La Bastide-van Gemert, S.; Maaike Vries, M. Long-term air pollution exposure, genome-wide DNA methylation and lung function in the LifeLines cohort study. Environ. Health Perspect. 2018, 126, 027004. [Google Scholar]
- Bergstra, A.D.; Brunekreef, B.; Burdorf, A. The effect of industry-related air pollution on lung function and respiratory symptoms in school children. Environ. Health 2018, 17, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, L.; Deng, F.; Tian, L.; Li, Y.; Swanson, M.; Ying, J.; Browne, R.W.; Rittenhouse-Olson, K.; Zhang, J.J.; Zhang, Z.-F. Peak expiratory flow, breath rate and blood pressure in adults with changes in particulate matter air pollution during the Beijing Olympics: A panel study. Environ. Res. 2014, 133, 4–11. [Google Scholar] [CrossRef] [Green Version]
- Sigaud, S.; Goldsmith, C.-A.W.; Zhou, H.; Yang, Z.; Fedulov, A.; Imrich, A.; Kobzik, L. Air pollution particles diminish bacterial clearance in the primed lungs of mice. Toxicol. Appl. Pharmacol. 2007, 223, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Sioutas, C.; Cho, A.; Schmitz, D.; Misra, C.; Sempf, J.; Wang, M.; Oberley, T.; Froines, J.; Nel, A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ. Health Perspect. 2003, 111, 455. [Google Scholar] [CrossRef]
- Diaz-Sanchez, D.; Proietti, L.; Polosa, R. Diesel fumes and the rising prevalence of atopy: An urban legend? Curr. Allergy Asthma Rep. 2003, 3, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Burchiel, S.W.; Lauer, F.T.; McDonald, J.D.; Reed, M.D. Systemic immunotoxicity in AJ mice following 6-month whole body inhalation exposure to diesel exhaust. Toxicol. Appl. Pharmacol. 2004, 196, 337–345. [Google Scholar] [CrossRef] [PubMed]
- van Eeden, S.F.; Hogg, J.C. Systemic inflammatory response induced by particulate matter air pollution: The importance of bone-marrow stimulation. J. Toxicol. Environ. Health Part A 2002, 65, 1597–1613. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Zhang, H.; Cai, Z.; Zhao, Y.; Wu, Y.; Zheng, X.; Liu, Y.; Qin, Y.; Gu, M.; Jin, J. Bufei huoxue capsule attenuates PM2.5-induced pulmonary inflammation in mice. Evid.-Based Complement. Altern. Med. 2017, 2017, 1575793. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.-H.; Lee, C.-L.; Su, H.-H.; Lee, C.-L.; Wu, C.-C.; Wang, C.-C.; Sheu, C.-C.; Lai, R.-S.; Leung, S.-Y.; Lin, C.-C. A prominent air pollutant, Indeno [1, 2, 3-cd] pyrene, enhances allergic lung inflammation via aryl hydrocarbon receptor. Sci. Rep. 2018, 8, 5198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Dai, Y.; Zhang, X.; Niu, Y.; Meng, T.; Li, Y.; Duan, H.; Bin, P.; Ye, M.; Jia, X. Reduced pulmonary function and increased pro-inflammatory cytokines in nanoscale carbon black-exposed workers. Part. Fibre Toxicol. 2014, 11, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, F.; Qiu, X.; Hu, X.; Shang, Y.; Pardo, M.; Fang, Y.; Wang, J.; Rudich, Y.; Zhu, T. Effects on IL-1β signaling activation induced by water and organic extracts of fine particulate matter (PM 2.5) in vitro. Environ. Pollut. 2018, 237, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Fernandez, L.G.; Awad, A.S.; Kron, I.L.; Laubach, V.E. Proinflammatory response of alveolar epithelial cells is enhanced by alveolar macrophage-produced TNF-α during pulmonary ischemia-reperfusion injury. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2007, 293, L105–L113. [Google Scholar] [CrossRef] [PubMed]
- Gossai, D.; Lau-Cam, C.A. The effects of taurine, taurine homologs and hypotaurine on cell and membrane antioxidative system alterations caused by type 2 diabetes in rat erythrocytes. Adv. Exp. Med. Biol. 2009, 643, 359–368. [Google Scholar]
- Bucak, M.N.; Tuncer, P.B.; Sarıözkan, S.; Ulutaş, P.A.; Çoyan, K.; Başpınar, N.; Özkalp, B. Effects of hypotaurine, cysteamine and aminoacids solution on post-thaw microscopic and oxidative stress parameters of Angora goat semen. Res. Vet. Sci. 2009, 87, 468–472. [Google Scholar] [CrossRef]
- Pradhan, M.P.; Desai, A.; Palakal, M.J. Systems biology approach to stage-wise characterization of epigenetic genes in lung adenocarcinoma. BMC Syst. Biol. 2013, 7, 141. [Google Scholar] [CrossRef]
- Navarova, H.; Bernsdorff, F.; Doring, A.C.; Zeier, J. Pipecolic Acid, an Endogenous Mediator of Defense Amplification and Priming, Is a Critical Regulator of Inducible Plant Immunity. Plant Cell 2012, 24, 5123–5141. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, R.; Lim, G.-H.; de Lorenzo, L.; Yu, K.; Zhang, K.; Hunt, A.G.; Kachroo, A.; Kachroo, P. Pipecolic acid confers systemic immunity by regulating free radicals. Sci. Adv. 2018, 4, eaar4509. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.-F. Pipecolic acid pathway: The major lysine metabolic route in the rat brain. Biochem. Biophys. Res. Commun. 1976, 69, 174–180. [Google Scholar] [CrossRef]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2011, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Bronte, V.; Zanovello, P. Regulation of immune responses by L-arginine metabolism. Nat. Rev. Immunol. 2005, 5, 641. [Google Scholar] [CrossRef] [PubMed]
- Pesce, J.T.; Ramalingam, T.R.; Mentink-Kane, M.M.; Wilson, M.S.; El Kasmi, K.C.; Smith, A.M.; Thompson, R.W.; Cheever, A.W.; Murray, P.J.; Wynn, T.A. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 2009, 5, e1000371. [Google Scholar] [CrossRef]
- Listed, N. Arginine metabolism: Enzymology, nutrition, and clinical significance. Proceedings of a symposium dedicated to the memory of Vernon R. Young. April 5–6, 2004. Bermuda. J. Nutr. 2004, 134, 2741S–2897S. [Google Scholar]
- Geiger, R.; Rieckmann, J.C.; Wolf, T.; Basso, C.; Feng, Y.; Fuhrer, T.; Kogadeeva, M.; Picotti, P.; Meissner, F.; Mann, M. L-arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell 2016, 167, 829–842. [Google Scholar] [CrossRef]
- Feun, L.; You, M.; Wu, C.; Kuo, M.; Wangpaichitr, M.; Spector, S.; Savaraj, N. Arginine deprivation as a targeted therapy for cancer. Curr. Pharm. Des. 2008, 14, 1049–1057. [Google Scholar] [CrossRef]
- Scott, J.; Chen, R.; North, M.; Urch, B.; Silverman, F.; Grasemann, H.; Chow, C.-W. Alterations of Arginine Metabolism and Lung Function Following Acute Exposure of Mice to Concentrated Ambient Fine Particulate Matter and Ozone. In D106. Cellular and Molecular Mechanisms of Environmental and Occupational Exposures; American Thoracic Society: New York, NY, USA, 2018; p. A7560. [Google Scholar]
- Kim, K.H.; Kim, E.J.; Kwun, M.J.; Lee, J.Y.; Bach, T.T.; Eum, S.M.; Choi, J.Y.; Cho, S.; Kim, S.-J.; Jeong, S.-I. Suppression of lung inflammation by the methanol extract of Spilanthes acmella Murray is related to differential regulation of NF-κB and Nrf2. J. Ethnopharmacol. 2018, 217, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Sun, Y.; Shen, Y.; Li, F.; Song, X.; Zhou, E.; Zhao, F.; Liu, Z.; Fu, Y.; Guo, M. Shikonin exerts anti-inflammatory effects in a murine model of lipopolysaccharide-induced acute lung injury by inhibiting the nuclear factor-kappaB signaling pathway. Int. Immunopharmacol. 2013, 16, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Kendall, M. Fine airborne urban particles (PM2.5) sequester lung surfactant and amino acids from human lung lavage. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 293, L1053–L1058. [Google Scholar] [CrossRef] [PubMed]
- Stoeger, T.; Reinhard, C.; Takenaka, S.; Schroeppel, A.; Karg, E.; Ritter, B.; Heyder, J.; Schulz, H. Instillation of six different ultrafine carbon particles indicates a surface area threshold dose for acute lung inflammation in mice. Environ. Health Perspect. 2005, 114, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Happle, C.; Lachmann, N.; Škuljec, J.; Wetzke, M.; Ackermann, M.; Brennig, S.; Mucci, A.; Jirmo, A.C.; Groos, S.; Mirenska, A. Pulmonary transplantation of macrophage progenitors as effective and long-lasting therapy for hereditary pulmonary alveolar proteinosis. Sci. Transl. Med. 2014, 6, 250ra113. [Google Scholar] [CrossRef] [PubMed]
- Hertz-Picciotto, I.; Herr, C.E.; Yap, P.-S.; Dostál, M.; Shumway, R.H.; Ashwood, P.; Lipsett, M.; Joad, J.P.; Pinkerton, K.E.; Šrám, R.J. Air pollution and lymphocyte phenotype proportions in cord blood. Environ. Health Perspect. 2005, 113, 1391. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhang, Z. Immunotoxicity of traffic related fine particles to human peripheral blood lymphocytes and calcium signal mechanism. J. Environ. Health 2010, 27, 946–949. [Google Scholar]
- Ma-Hock, L.; Farias, P.M.; Hofmann, T.; Andrade, A.C.; Silva, J.N.; Arnaud, T.M.; Wohlleben, W.; Strauss, V.; Treumann, S.; Chaves, C. Short term inhalation toxicity of a liquid aerosol of glutaraldehyde-coated CdS/Cd(OH)2 core shell quantum dots in rats. Toxicol. Lett. 2014, 225, 20–26. [Google Scholar] [CrossRef]
- Fang, Z.-Z.; Krausz, K.W.; Tanaka, N.; Li, F.; Qu, A.; Idle, J.R.; Gonzalez, F.J. Metabolomics reveals trichloroacetate as a major contributor to trichloroethylene-induced metabolic alterations in mouse urine and serum. Arch. Toxicol. 2013, 87, 1975–1987. [Google Scholar] [CrossRef]
- Fang, Z.-Z.; Tanaka, N.; Lu, D.; Jiang, C.-T.; Zhang, W.-H.; Zhang, C.; Du, Z.; Fu, Z.-W.; Gao, P.; Cao, Y.-F. Role of the lipid-regulated NF-κB/IL-6/STAT3 axis in alpha-naphthyl isothiocyanate-induced liver injury. Arch. Toxicol. 2017, 91, 2235–2244. [Google Scholar] [CrossRef]







| Group | Colla corii asini (g/kg) | Number | CD3+ (%) | CD4+ (%) | CD8+ (%) | CD4+/CD8+ |
|---|---|---|---|---|---|---|
| Control | - | 8 | 55.29 ± 9.46 | 44.63 ± 8.89 | 10.26 ± 3.31 | 4.84 ± 2.06 |
| aPM2.5 | - | 8 | 54.69 ± 5.52 | 44.44 ± 5.50 | 10.16 ± 4.56 | 5.54 ± 3.09 |
| Low-dose group | 1 | 8 | 51.28 ± 5.26 | 44.36 ± 5.49 | 6.18 ± 2.55 #,* | 8.48 ± 3.70 # |
| Mid-dose group | 2 | 8 | 58.44 ± 8.44 | 48.14 ± 10.1 | 7.01 ± 4.83 | 8.22 ± 3.47 # |
| High-dose group | 5 | 8 | 54.18 ± 4.79 | 47.07 ± 5.08 | 6.79 ± 2.93 | 8.95 ± 5.98 # |
| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| TNF-α | ACCAGCAGATGGGCTGTA | CTCCTGGTATGAAATGGCAA |
| IL-1β | CAAGGAGAGACAAGCAACG | TTCATCACACAGGACAGGTAT |
| IL-10 | ACTGGACTGCAGGACATA | TTTGGAGAGAGGTACAAACGA |
| iNOS | TTGCTTCTGTGCTAATGCG | TCCGACTTTCCTGTCTCAGTA |
| Arg-1 | TCGTACTGTGAACACGGC | GGTAGTCAGTCTCTGGCTTAT |
| ACTB | CCACCATGTACCCAGGCATT | CGGACTCATCGTACTCCTGC |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Zhang, P.; Ling, Y.; Hu, G.; Gu, J.; Yang, H.; Wei, J.; Wang, A.; Jin, H. Protective Effect of Colla corii asini against Lung Injuries Induced by Intratracheal Instillation of Artificial Fine Particles in Rats. Int. J. Mol. Sci. 2019, 20, 55. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20010055
Liu T, Zhang P, Ling Y, Hu G, Gu J, Yang H, Wei J, Wang A, Jin H. Protective Effect of Colla corii asini against Lung Injuries Induced by Intratracheal Instillation of Artificial Fine Particles in Rats. International Journal of Molecular Sciences. 2019; 20(1):55. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20010055
Chicago/Turabian StyleLiu, Tiantian, Piaopiao Zhang, Yahao Ling, Guang Hu, Jianjun Gu, Hong Yang, Jinfeng Wei, Aiping Wang, and Hongtao Jin. 2019. "Protective Effect of Colla corii asini against Lung Injuries Induced by Intratracheal Instillation of Artificial Fine Particles in Rats" International Journal of Molecular Sciences 20, no. 1: 55. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20010055