Epithelial to Mesenchymal Transition in Human Mesothelial Cells Exposed to Asbestos Fibers: Role of TGF-β as Mediator of Malignant Mesothelioma Development or Metastasis via EMT Event
Abstract
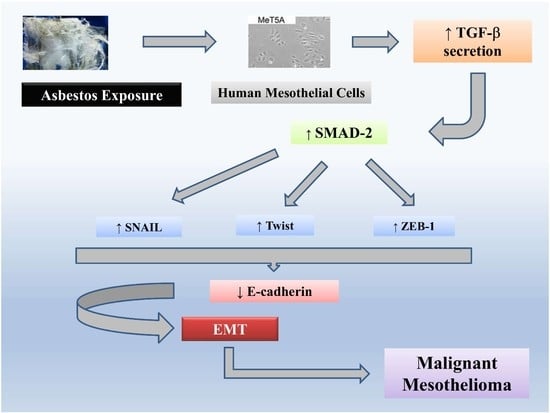
:1. Introduction
2. Results
2.1. Asbestos Fibers Induce Fibroblastoid Morphological Changes in MeT-5A Cells
2.2. Chrysotile Asbestos Downregulates Epithelial Markers and Upregulates Mesenchymal Markers in MeT-5A Cells
2.3. Chrysotile Increases MMP-2 Secretion While EMT Event Was Induced
2.4. Exposure to Chrysotile Asbestos Increases TGF-β Secretion in MeT-5A Cells and Co-Incubation with Anti-TGF-β Antibody Restores Basal Expression Level of EMT Markers
2.5. Exposure to Chrysotile Induces E-Cadherin Downregulation Through SMAD Pathway via Increased Secretion of TGF-β
2.6. EMT Induced by Chrysotile is Mediated by GSK-3β/SNAIL-1 Activation
2.7. Twist and ZEB-1 Factors Are Involved in EMT via TGF-β in MeT-5A Cells Exposed to Asbestos Fibers
3. Discussion
4. Materials and Methods
4.1. Asbestos Samples
4.2. Cell Cultures
4.3. Experimental Conditions
4.4. Cell Morphology
4.5. Western Blot Analysis
4.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.7. Quantification of TGF-β Secretion by ELISA
4.8. Gelatin Zymography
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kamp, D.W. Asbestos-induced lung diseases: An update. Transl. Res. 2009, 153, 143–152. [Google Scholar] [CrossRef]
- Kamp, D.W.; Weitzman, S.A. The molecular basis of asbestos induced lung injury. Thorax 1999, 54, 638–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalluri, R.; Neilson, E.G. Epithelial-mesenchymal transition and its implications for fibrosis. J. Clin. Investig. 2003, 112, 1776–1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2018, 20. [Google Scholar] [CrossRef] [PubMed]
- Cannito, S.; Novo, E.; Di Bonzo, L.V.; Busletta, C.; Colombatto, S.; Parola, M. Epithelial-mesenchymal transition: From molecular mechanisms, redox regulation to implications in human health and disease. Antioxid. Redox Signal 2010, 12, 1383–1430. [Google Scholar] [CrossRef] [PubMed]
- Boyer, B.; Vallés, A.M.; Edme, N. Induction and regulation of epithelial-mesenchymal transitions. Biochem. Pharmacol. 2000, 60, 1091–1099. [Google Scholar] [CrossRef]
- Peinado, H.; Portillo, F.; Cano, A. Transcriptional regulation of cadherins during development and carcinogenesis. Int. J. Dev. Biol. 2004, 48, 365–375. [Google Scholar] [CrossRef] [Green Version]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [Green Version]
- Moustakas, A.; Heldin, C.H. Induction of epithelial-mesenchymal transition by transforming growth factor β. Semin. Cancer Biol. 2012, 22, 446–454. [Google Scholar] [CrossRef]
- Chen, J.; Chen, G.; Yan, Z.; Guo, Y.; Yu, M.; Feng, L.; Jiang, Z.; Guo, W.; Tian, W. TGF-β1 and FGF2 stimulate the epithelial-mesenchymal transition of HERS cells through a MEK-dependent mechanism. J. Cell Physiol. 2014, 229, 1647–1659. [Google Scholar] [CrossRef] [PubMed]
- Farrell, J.; Kelly, C.; Rauch, J.; Kida, K.; García-Muñoz, A.; Monsefi, N.; Turriziani, B.; Doherty, C.; Mehta, J.P.; Matallanas, D.; et al. HGF induces epithelial-to-mesenchymal transition by modulating the mammalian Hippo/MST2 and ISG15 pathways. J. Proteome Res. 2014, 13, 2874–2886. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, N.A.; Ghiassi, M.; Bakin, A.; Aakre, M.; Lundquist, C.A.; Engel, M.E.; Arteaga, C.L.; Moses, H.L. Transforming growth factor-beta1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol. Biol. Cell 2001, 12, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Tsubakihara, Y.; Moustakas, A. Epithelial-Mesenchymal Transition and Metastasis under the Control of Transforming Growth Factor β. Int. J. Mol. Sci. 2018, 19, 3672. [Google Scholar] [CrossRef] [PubMed]
- Manning, C.B.; Vallyathan, V.; Mossman, B.T. Diseases caused by asbestos: Mechanism of injury and disease development. Int. Immunopharmacol. 2002, 2, 191–200. [Google Scholar] [CrossRef]
- Tamminen, J.A.; Myllärniemi, M.; Hyytiäinen, M.; Keski-Oja, J.; Koli, K. Asbestos exposure induces alveolar epithelial cell plasticity through MAPK/Erk signaling. J. Cell Biochem. 2012, 113, 2234–2247. [Google Scholar] [CrossRef]
- Qi, F.; Okimoto, G.; Jube, S.; Napolitano, A.; Pass, H.I.; Laczko, R.; Demay, R.M.; Khan, G.; Tiirikainen, M.; Rinaudo, C.; et al. Continuous exposure to chrysotile asbestos can cause transformation of human mesothelial cells via HMGB1 and TNF-α signaling. Am. J. Pathol. 2013, 183, 1654–1666. [Google Scholar] [CrossRef]
- Pociask, D.A.; Sime, P.J.; Brody, A.R. Asbestos-derived reactive oxygen species activate TGF-beta1. Lab. Investig. 2004, 84, 1013–1023. [Google Scholar] [CrossRef]
- Maeda, M.; Chen, Y.; Hayashi, H.; Kumagai-Takei, N.; Matsuzaki, H.; Lee, S.; Nishimura, Y.; Otsuki, T. Chronic exposure to asbestos enhances TGF-β1 production in the human adult T cell leukemia virus-immortalized T cell line MT-2. Int. J. Oncol. 2014, 45, 2522–2532. [Google Scholar] [CrossRef]
- Gulino, G.R.; Polimeni, M.; Prato, M.; Gazzano, E.; Kopecka, J.; Colombatto, S.; Ghigo, D.; Aldieri, E. Effects of Chrysotile Exposure in Human Bronchial Epithelial Cells: Insights into the Pathogenic Mechanisms of Asbestos-Related Diseases. Environ. Health Perspect. 2016, 124, 776–784. [Google Scholar] [CrossRef] [Green Version]
- Xie, F.; Zhang, Z.; Van Dam, H.; Zhang, L.; Zhou, F. Regulation of TGFβ Superfamily Signalling by SMAD Mono-Ubiquitination. Cell 2014, 3, 981–993. [Google Scholar] [CrossRef] [PubMed]
- Casarsa, C.; Bassani, N.; Ambrogi, F.; Zabucchi, G.; Boracchi, P.; Biganzoli, E.; Coradini, D. Epithelial-to-mesenchymal transition, cell polarity and stemness-associated features in malignant pleural mesothelioma. Cancer Lett. 2011, 302, 136–143. [Google Scholar] [CrossRef]
- Schramm, A.; Opitz, I.; Thies, S.; Seifert, B.; Moch, H.; Weder, W.; Soltermann, A. Prognostic significance of epithelial-mesenchymal transition in malignant pleural mesothelioma. Eur. J. Cardiothorac. Surg. 2010, 37, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.C.; Hwang, S.H.; Kim, N.Y.; Lee, H.S.; Ji, S.; Yang, Y.; Kim, Y. Hypoxia promotes acquisition of aggressive phenotypes in human malignant mesothelioma. BMC Cancer 2018, 18, 819. [Google Scholar] [CrossRef]
- Katoh, M.; Katoh, M. Cross-talk of WNT and FGF signaling pathways at GSK3beta to regulate beta-catenin and SNAIL signaling cascades. Cancer Biol. Ther. 2006, 5, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.P.; Deng, J.; Xia, W.; Xu, J.; Li, Y.M.; Gunduz, M.; Hung, M.C. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat. Cell Biol. 2004, 6, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Olmeda, D.; Cano, A. Snail, Zeb and bHLH factors in tumour progression: An alliance against the epithelial phenotype? Nat. Rev. Cancer 2007, 7, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.E.; Ferris, M.; Pociask, D.; Brody, A.R. The latent form of TGFbeta(1) is induced by TNFalpha through an ERK specific pathway and is activated by asbestos-derived reactive oxygen species in vitro and in vivo. J. Immunotoxicol. 2008, 5, 145–149. [Google Scholar] [CrossRef]
- Miyazono, K. Transforming growth factor-beta signaling in epithelial-mesenchymal transition and progression of cancer. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2009, 85, 314–323. [Google Scholar] [CrossRef]
- Jope, R.S.; Yuskaitis, C.J.; Beurel, E. Glycogen synthase kinase-3 (GSK3): Inflammation, diseases, and therapeutics. Neurochem. Res. 2007, 32, 577–595. [Google Scholar] [CrossRef]
- Merikallio, H.; Pääkkö, P.; Salmenkivi, K.; Kinnula, V.; Harju, T.; Soini, Y. Expression of snail, twist, and Zeb1 in malignant mesothelioma. APMIS 2013, 121, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Horie, M.; Nagase, T. TGF-β Signaling in Lung Health and Disease. Int. J. Mol. Sci. 2018, 19, 2460. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Horie, M.; Micke, P.; Nagase, T. The Role of TGF-β Signaling in Lung Cancer Associated with Idiopathic Pulmonary Fibrosis. Int. J. Mol. Sci. 2018, 19, 3611. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, B.; Schwerdtle, T.; Poser, I.; Hoffmann, E.; Hartwig, A.; Müller, W.U.; Rettenmeier, A.W.; Seemayer, N.H.; Dopp, E. Effects of asbestos on initiation of DNA damage, induction of DNA-strand breaks, P53-expression and apoptosis in primary, SV40-transformed and malignant human mesothelial cells. Mutat. Res. 2004, 558, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Simeone, P.; Trerotola, M.; Franck, J.; Cardon, T.; Marchisio, M.; Fournier, I.; Salzet, M.; Maffia, M.; Vergara, D. The multiverse nature of epithelial to mesenchymal transition. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Giribaldi, G.; Valente, E.; Khadjavi, A.; Polimeni, M.; Prato, M. Macrophage inflammatory protein-1alpha mediates matrix metalloproteinase-9 enhancement in human adherent monocytes fed with malarial pigment. Asian Pac. J. Trop. Med. 2011, 4, 925–930. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turini, S.; Bergandi, L.; Gazzano, E.; Prato, M.; Aldieri, E. Epithelial to Mesenchymal Transition in Human Mesothelial Cells Exposed to Asbestos Fibers: Role of TGF-β as Mediator of Malignant Mesothelioma Development or Metastasis via EMT Event. Int. J. Mol. Sci. 2019, 20, 150. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20010150
Turini S, Bergandi L, Gazzano E, Prato M, Aldieri E. Epithelial to Mesenchymal Transition in Human Mesothelial Cells Exposed to Asbestos Fibers: Role of TGF-β as Mediator of Malignant Mesothelioma Development or Metastasis via EMT Event. International Journal of Molecular Sciences. 2019; 20(1):150. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20010150
Chicago/Turabian StyleTurini, Stefano, Loredana Bergandi, Elena Gazzano, Mauro Prato, and Elisabetta Aldieri. 2019. "Epithelial to Mesenchymal Transition in Human Mesothelial Cells Exposed to Asbestos Fibers: Role of TGF-β as Mediator of Malignant Mesothelioma Development or Metastasis via EMT Event" International Journal of Molecular Sciences 20, no. 1: 150. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20010150