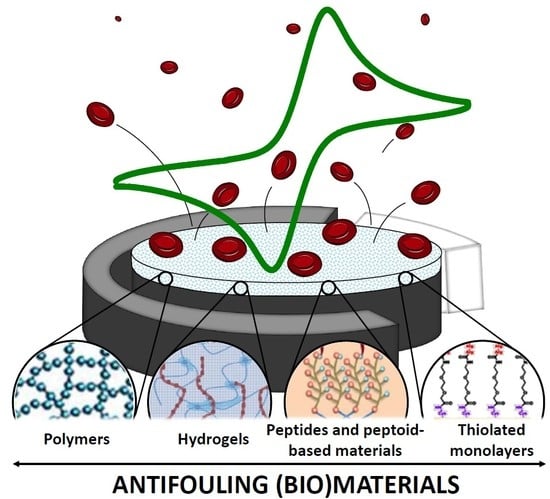
Antifouling (Bio)materials for Electrochemical (Bio)sensing
Abstract
:1. Introduction
2. Antibiofouling Polymers
2.1. PEG Polymers
2.2. Conducting Polymers
2.3. Zwitterionic Polymers
2.4. pH-Responsive Methacrylate Polymers
3. Antibiofouling Hydrogels
4. Antibiofouling Peptides and Peptoid-Based Materials
5. Antibiofouling Thiolated Monolayers
5.1. Thioaromatic DNA Monolayers
5.2. Ternary DNA Monolayers
5.3. PEG-based SAMs
5.4. Tetrahedral DNA Nanostructures
5.5. Other Thiolated SAMs
6. General Conclusions and Personal Viewpoints
Funding
Conflicts of Interest
Abbreviations
| AFP | alphafetoprotein |
| p-ATP | p-aminothiophenol |
| AuNPs | gold nanoparticles |
| B-IFN-γ | bovine-interferon-γ |
| BSA | bovine serum albumin |
| CEA | carcinoembryonic antigen |
| CV | cyclic voltammetry |
| DNA | deoxyribonucleic acid |
| DPV | differential pulse voltammetry |
| DTT | DL-dithiothreitol |
| EDC | 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide |
| EDOT | 3,4-ethylenedioxythiophene |
| E-PB | electrochemical peptide-based |
| GI | gastrointestinal |
| GCE | glassy carbon electrode |
| GOx | glucose oxidase |
| HDA | helicase-dependent amplification |
| HDT | 1,6-hexanedithiol |
| HNE | human neutrophil elastase |
| IgE | immunoglobulin E |
| LOD | limit of detection |
| MB | methylene blue |
| p-MBA | p-mercaptobenzoic acid |
| MCH | mercaptohexanol |
| MEA | monoethanolamine |
| MPA | 3-mercaptopropionic acid |
| MPC | 2-methacryloyloxyethyl phosphorylcholine |
| NaPSS | poly(sodium-4-styrenesulfonate) |
| NDT | 1,9-nonanedithiol |
| NHS | N-hydroxysuccinimide |
| PANI | polyaniline |
| pCBMA | polycarboxybetaine methacrylate |
| PCR | polymerase chain reaction |
| PDT | 1,3-propanedithiol |
| PEDOT:PSS | poly(3,4-ethylenedioxythiophene)-poly(styrene sulfonate) |
| PEG | poly(ethylene glycol) |
| PB | prussian blue |
| PSA | prostate specific antigen |
| pSBMA | polysulfobetaine methacrylate |
| PspA | pneumococcal surface protein A |
| QSPR | Quantitative Structure-Property Relationship |
| RNA | ribonucleic acid |
| rRNA | ribosomal RNA |
| SAMs | self-assembled monolayers |
| SHCP | thiolated capture probe |
| S/N | signal-to-noise |
| ssDNA | single-stranded DNA |
| SWV | square wave voltammetry |
| TCP | tricresyl phosphate |
References
- Yanga, X.; Kirscha, J.; Olsen, E.V.; Fergusa, J.W.; Simoniana, A.L. Anti-fouling PEDOT:PSS modification on glassy carbon electrodes for continuous monitoring of tricresyl phosphate. Sens. Actuators B Chem. 2013, 177, 659–667. [Google Scholar] [CrossRef]
- Hanssen, B.L.; Siraj, S.; Wong, D.K.Y. Recent strategies to minimise fouling in electrochemical detection systems. Rev. Anal. Chem. 2016, 35, 1–28. [Google Scholar] [CrossRef]
- Hui, N.; Sun, X.; Niu, S.; Luo, X. PEGylated polyaniline nanofibers: Antifouling and conducting biomaterial for electrochemical DNA sensing. ACS Appl. Mater. Interfaces 2017, 9, 2914–2923. [Google Scholar] [CrossRef] [PubMed]
- Blaszykowski, C.; Sheikh, S.; Thompson, M. Surface chemistry to minimize fouling from blood-based fluids. Chem. Soc. Rev. 2012, 41, 5599–5612. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, M.; Jiao, M.; Luo, X. Antifouling and ultrasensitive biosensing interface based on self-assembled peptide and aptamer on macroporous gold for electrochemical detection of immunoglobulin E in serum. Anal. Bioanal. Chem. 2018, 410, 5871–5878. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Wang, Y.; Jiao, M.; Jayachandran, S.; Wu, Y.; Fan, X.; Luo, X. Mixed self-assembled aptamer and newly designed zwitterionic peptide as antifouling biosensing interface for electrochemical detection of alpha-fetoprotein. ACS Sens. 2017, 2, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Damodaran, V.B.; Murthy, N.S. Bio-inspired strategies for designing antifouling biomaterials. Biomater. Res. 2016, 20, 18. [Google Scholar] [CrossRef]
- Francolini, I.; Vuotto, C.; Piozzi, A.; Donelli, G. Antifouling and antimicrobial biomaterials: An overview. APMIS 2017, 125, 392–417. [Google Scholar] [CrossRef]
- Abbina, S.; Vappala, S.; Kumar, P.; Siren, E.M.J.; La, C.C.; Abbasi, U.; Brooks, D.E.; Kizhakkedathu, J.N. Hyperbranched polyglycerols: Recent advances in synthesis, biocompatibility and biomedical applications. J. Mater. Chem. B 2017, 5, 9249–9277. [Google Scholar] [CrossRef]
- Luk, Y.Y.; Kato, M.; Mrksich, M. Self-assembled monolayers of alkanethiolates presenting mannitol groups are inert to protein adsorption and cell attachment. Langmuir 2000, 16, 9604–9608. [Google Scholar] [CrossRef]
- Snyder, T.A.; Tsukui, H.; Kihara, S.; Akimoto, T.; Litwak, K.N.; Kameneva, M.V.; Yamazaki, K.; Wagner, W.R. Preclinical biocompatibility assessment of the EVAHEART ventricular assist device: Coating comparison and platelet activation. J. Biomed. Mater. Res. Part A 2007, 81, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Goda, T.; Tabata, M.; Sanjoh, M.; Uchimura, M.; Iwasaki, Y.; Miyahara, Y. Thiolated 2-methacryloyloxyethyl phosphorylcholine for an antifouling biosensor platform. Chem. Commun. 2013, 49, 8683–8685. [Google Scholar] [CrossRef] [PubMed]
- Lowe, S.; O’Brien-Simpson, N.M.; Connal, L.A. Antibiofouling polymer interfaces: poly(ethylene glycol) and other promising candidates. Polym. Chem. 2015, 6, 198–212. [Google Scholar] [CrossRef]
- Walker, J.A.; Robinson, K.J.; Munro, C.; Gengenbach, T.; Muller, D.A.; Young, P.R.; Lua, L.H.L.; Corrie, S.R. Antibody-binding, antifouling surface coatings based on recombinant expression of zwitterionic EK peptides. Langmuir 2018. [Google Scholar] [CrossRef] [PubMed]
- Walper, S.A.; Lee, P.A.B.; Goldman, E.R.; Anderson, G.P. Comparison of single domain antibody immobilization strategies evaluated by surface plasmon resonance. J. Immunol. Methods 2013, 388, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Welch, N.G.; Scoble, J.A.; Muir, B.W.; Pigram, P.J. Orientation and characterization of immobilized antibodies for improved immunoassays. Biointerphases 2017, 12, 02D301. [Google Scholar] [CrossRef]
- Gölander, C.-G.; Herron, J.N.; Lim, K.; Claesson, P.; Stenius, P.; Andrade, J.D. Properties of Immobilized PEG Films and the Interaction with Proteins, in poly(ethylene glycol) Chemistry; Springer: Berlin, Germany, 1992; pp. 221–245. [Google Scholar]
- Lüsse, S.; Arnold, K. The interaction of poly(ethylene glycol) with water studied by 1H and 2H NMR relaxation time measurements. Macromolecules 1996, 29, 4251–4257. [Google Scholar] [CrossRef]
- Oesterhelt, F.; Rief, M.; Gaub, H. Single molecule force spectroscopy by AFM indicates helical structure of poly(ethylene-glycol) in water. New J. Phys. 1999, 1, 6. [Google Scholar] [CrossRef]
- Aray, Y.; Marquez, M.; Rodríguez, J.; Vega, D.; Simón-Manso, Y.; Coll, S.; Gonzalez, C.; Weitz, D.A. Electrostatics for exploring the nature of the hydrogen bonding in polyethylene oxide hydration. J. Phys. Chem. B 2004, 108, 2418–2424. [Google Scholar] [CrossRef]
- Kingshott, P.; Wei, J.; Bagge-Ravn, D.; Gadegaard, N.; Gram, L. Covalent attachment of poly(ethylene glycol) to surfaces, critical for reducing bacterial adhesion. Langmuir 2003, 19, 6912–6921. [Google Scholar] [CrossRef]
- Damodaran, V.B.; Fee, C.J.; Popat, K.C. Prediction of protein interaction behaviour with PEG-grafted matrices using X-ray photoelectron spectroscopy. Appl. Surf. Sci. 2010, 256, 4894–4901. [Google Scholar] [CrossRef]
- Ostuni, E.; Chapman, R.G.; Erik Holmlin, R.; Takayama, S.; Whitesides, G.M. A survey of structure–property relationships of surfaces that resist the adsorption of protein. Langmuir 2001, 17, 5605–5620. [Google Scholar] [CrossRef]
- Patra, S.; Munichandraiah, N. Electro-oxidation of phenol on polyethylenedioxythiophene conductive-polymer-deposited stainless steel substrate. J. Electrochem. Soc. 2008, 155, F23–F30. [Google Scholar] [CrossRef]
- Mark, H.B.; Atta, N.; Ma, Y.L.; Petticrew, K.L.; Zimmer, H.; Shi, Y.; Lunsford, S.K.; Rubinson, J.F.; Galal, A. The electrochemistry of neurotransmitters at conducting organic polymer electrodes: Electrocatalysis and analytical applications. Bioelectrochem. Bioenerg. 1995, 38, 229–245. [Google Scholar] [CrossRef]
- Malinauskas, A. Electrocatalysis at conducting polymers. Synth. Met. 1999, 107, 75–83. [Google Scholar] [CrossRef]
- Rosatto, S.S.; Kubota, L.T.; de Oliveira Neto, G. Biosensor for phenol based on the direct electron transfer blocking of peroxidase immobilising on silica–titanium. Anal. Chim. Acta 1999, 390, 65–72. [Google Scholar] [CrossRef]
- Lupu, S.; Mucci, A.; Pigani, L.; Seeber, R.; Zanardi, C. Polythiophene derivative conducting polymer modified electrodes and microelectrodes for determination of ascorbic acid. Effect of possible interferents. Electroanalysis 2002, 14, 519–525. [Google Scholar] [CrossRef]
- Sun, X.; Wang, H.; Wang, Y.; Gui, T.; Wang, K.; Gao, C. Creation of antifouling microarrays by photopolymerization of zwitterionic compounds for protein assay and cell patterning. Biosens. Bioelectron. 2018, 102, 63–69. [Google Scholar] [CrossRef]
- Ruiz-Valdepeñas Montiel, V.; Sempionatto, J.R.; Esteban-Fernández de Ávila, B.; Whitworth, A.; Campuzano, S.; Pingarrón, J.M.; Wang, J. Delayed sensor activation based on transient coatings: Biofouling protection in complex biofluids. J. Am. Chem. Soc. 2018, 140, 14050–14053. [Google Scholar] [CrossRef]
- Ruiz-Valdepeñas Montiel, V.; Sempionatto, J.R.; Campuzano, S.; Pingarrón, J.M.; Esteban-Fernández de Ávila, B.; Wang, J. Direct electrochemical biosensing in gastrointestinal fluids. Anal. Bioanal. Chem. 2018. [Google Scholar] [CrossRef]
- Tavakoli, J.; Tang, Y. Hydrogel Based Sensors for Biomedical Applications: An Updated Review. Polymers 2017, 9, 364. [Google Scholar] [CrossRef]
- Rong, Q.; Han, H.; Feng, F.; Ma, Z. Network nanostructured polypyrrole hydrogel/Au composites as enhanced electrochemical biosensing platform. Sci. Rep. 2015, 5, 11440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, D.-S.; Matharu, Z.; You, J.; Siltanen, C.; Vu, T.; Raghunathan, V.K.; Stybayeva, G.; Hill, A.E.; Revzin, A. Sensing conductive hydrogels for rapid detection of cytokines in blood. Adv. Healthc. Mater. 2016, 5, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Leng, C.; Buss, H.G.; Segalman, R.A.; Chen, Z. Surface structure and hydration of sequence-specific amphiphilic polypeptoids for antifouling/fouling release applications. Langmuir 2015, 31, 9306–9311. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cheng, F.; Shen, W.; Cheng, G.; Zhao, J.; Peng, W.; Qu, J. Amino acid-based anti-fouling functionalization of silica nanoparticles using divinyl sulfone. Acta Biomater. 2016, 40, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Chelmowski, R.; Köster, S.D.; Kerstan, A.; Prekelt, A.; Grunwald, C.; Winkler, T.; Metzler-Nolte, N.; Terfort, A.; Wöll, C. Peptide-based SAMs that resist the adsorption of proteins. J. Am. Chem. Soc. 2008, 130, 14952–14953. [Google Scholar] [CrossRef]
- Nowinski, A.K.; Sun, F.; White, A.D.; Keefe, A.J.; Jiang, S. Sequence, structure, and function of peptide self-assembled monolayers. J. Am. Chem. Soc. 2012, 134, 6000–6005. [Google Scholar] [CrossRef]
- Keefe, A.J.; Caldwell, K.B.; Nowinski, A.K.; White, A.D.; Thakkar, A.; Jiang, S. Screening nonspecific interactions of peptides without background interference. Biomaterials 2013, 34, 1871–1877. [Google Scholar] [CrossRef] [Green Version]
- van Zoelen, W.; Buss, H.G.; Ellebracht, N.C.; Lynd, N.A.; Fischer, D.A.; Finlay, J.; Hill, S.; Callow, M.E.; Callow, J.A.; Kramer, E.J.; et al. Sequence of hydrophobic and hydrophilic residues in amphiphilic polymer coatings affects surface structure and marine antifouling/fouling release properties. ACS Macro Lett. 2014, 3, 364–368. [Google Scholar] [CrossRef]
- Wang, G.; Han, R.; Sua, X.; Lia, Y.; Xua, G.; Luo, X. Zwitterionic peptide anchored to conducting polymer PEDOT for the development of antifouling and ultrasensitive electrochemical DNA sensor. Biosens. Bioelectron. 2017, 92, 396–401. [Google Scholar] [CrossRef]
- Paleček, E.; Bartošík, M. Electrochemistry of nucleic acids. Chem. Rev. 2012, 112, 3427–3481. [Google Scholar] [CrossRef] [PubMed]
- Chaki, N.K.; Vijayamohanan, K. Self-assembled monolayers as a tunable platform for biosensor applications. Biosens. Bioelectron. 2002, 17, 1–12. [Google Scholar] [CrossRef]
- Aufartova, J.; Sánchez-Paniagua, L.M.; Martín-Fernández, B.; López-Ruiz, B. Key factors of ternary monolayers to improve DNA sensors performance. Electroanalysis 2017, 29, 131–139. [Google Scholar] [CrossRef]
- Herne, T.M.; Tarlov, M.J. Characterization of DNA probes immobilized on gold surfaces. J. Am. Chem. Soc. 1997, 119, 8916–8920. [Google Scholar] [CrossRef]
- Steel, A.B.; Herne, T.M.; Tarlov, M.J. Electrochemical quantitation of DNA immobilized on gold. Anal. Chem. 1998, 70, 4670–4677. [Google Scholar] [CrossRef] [PubMed]
- Levicky, R.; Herne, T.; Tarlov, M.; Satija, S. Using self-assembly to control the structure of DNA monolayers on gold: A neutron reflectivity study. J. Am. Chem. Soc. 1998, 120, 9787–9792. [Google Scholar] [CrossRef]
- Lao, R.J.; Song, S.P.; Wu, H.P.; Wang, L.H.; Zhang, Z.Z.; He, L.; Fan, C.H. Electrochemical interrogation of DNA monolayers on gold surfaces. Anal. Chem. 2005, 77, 6475–6480. [Google Scholar] [CrossRef]
- Keighley, S.D.; Li, P.; Estrela, P.; Mighorato, P. Optimization of DNA immobilization on gold electrodes for label-free detection by electrochemical impedance spectroscopy. Biosens. Bioelectron. 2008, 23, 1291–1297. [Google Scholar] [CrossRef]
- Boozer, C.; Chen, S.F.; Jiang, S.Y. Controlling DNA orientation on mixed ssDNA/OEG SAMs. Langmuir 2006, 22, 4694–4698. [Google Scholar] [CrossRef]
- Dharuman, V.; Hahn, J.H. Label free electrochemical DNA hybridization discrimination effects at the binary and ternary mixed monolayers of single stranded DNA/diluent/s in presence of cationic intercalators. Biosens. Bioelectron. 2008, 23, 1250–1258. [Google Scholar] [CrossRef]
- Wu, J.; Campuzano, S.; Halford, C.; Haake, D.A.; Wang, J. Ternary surface monolayers for ultrasensitive (zeptomole) amperometric detection of nucleic acid hybridization without signal amplification. Anal. Chem. 2010, 82, 8830–8837. [Google Scholar] [CrossRef] [PubMed]
- Campuzano, S.; Kuralay, F.; Lobo-Castañón, M.J.; Bartošik, M.; Vyavahare, K.; Paleček, E.; Haake, D.A.; Wang, J. Ternary monolayers as DNA recognition interfaces for direct and sensitive electrochemical detection in untreated clinical samples. Biosens. Bioelectron. 2011, 26, 3577–3583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Josephs, E.A.; Ye, T. A Single-Molecule View of conformational switching of DNA tethered to a gold electrode. J. Am. Chem. Soc. 2012, 134, 10021–10030. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Castro, R.; Sánchez-Salcedo, R.; Suárez-Álvarez, B.; de-los-Santos-Álvarez, N.; Miranda-Ordieres, A.J.; Lobo-Castañón, M.J. Thioaromatic DNA monolayers for target-amplification-free electrochemical sensing of environmental pathogenic bacteria. Biosens. Bioelectron. 2017, 92, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Castro, R.; de-los-Santos-Álvarez, N.; Lobo-Castañón, M.J. Understanding the factors affecting the analytical performance of sandwich-hybridization genosensors on gold electrodes. Electroanalysis 2018, 30, 1229–1240. [Google Scholar] [CrossRef]
- Campuzano, S.; Kuralay, F.; Wang, J. Ternary monolayer interfaces for ultrasensitive and direct bioelectronics detection of nucleic acids in complex matrices. Electroanalysis 2012, 24, 483–493. [Google Scholar] [CrossRef]
- González-Fernández, E.; Staderini, M.; Avlonitis, N.; Murray, A.F.; Mount, A.R.; Bradley, M. Effect of spacer length on the performance of peptide-based electrochemical biosensors for protease detection. Sens. Actuators B Chem. 2018, 255, 3040–3046. [Google Scholar] [CrossRef]
- Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Current trends and challenges in bioelectrochemistry for non-invasive and early diagnosis. Curr. Opin. Electrochem. 2018, 12, 81–91. [Google Scholar] [CrossRef]
- Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Tailoring sensitivity in electrochemical nucleic acid hybridization biosensing: Role of surface chemistry and labeling strategies. ChemElectroChem 2019, 6, 60–72. [Google Scholar] [CrossRef]
- Sabatani, E.; Cohen-Boulakia, J.; Bruening, M.; Rubinstein, I. Thioaromatic monolayers on gold: A new family of self-assembling monolayers. Langmuir 1993, 9, 2974–2981. [Google Scholar] [CrossRef]
- Hayes, W.A.; Shannon, C. Electrochemistry of surface-confined mixed monolayers of 4-aminothiophenol and thiophenol on Au. Langmuir 1996, 12, 3688–3694. [Google Scholar] [CrossRef]
- Moura-Melo, S.; Miranda-Castro, R.; de-los-Santos-Álvarez, N.; Miranda-Ordieres, A.J.; Dos Santos Junior, J.R.; da Silva Fonseca, R.A.; Lobo-Castañón, M.J. Targeting helicase-dependent amplification products with an electrochemical genosensor for reliable and sensitive screening of genetically modified organisms. Anal. Chem. 2015, 87, 8547–8554. [Google Scholar] [CrossRef] [PubMed]
- Kuralay, F.; Campuzano, S.; Haake, D.A.; Wang, J. Highly sensitive disposable nucleic acid biosensors for direct bioelectronic detection in raw biological samples. Talanta 2011, 85, 1330–1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuralay, F.; Campuzano, S.; Wang, J. Greatly extended storage stability of electrochemical DNA biosensors using ternary thiolated self-assembled monolayers. Talanta 2012, 99, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Halford, C.; Gonzalez, R.; Campuzano, S.; Hu, B.; Babbitt, J.T.; Liu, J.; Wang, J.; Churchill, B.M.; Haake, D.A. Rapid antimicrobial susceptibility testing by sensitive detection of precursor rRNA using a novel electrochemical biosensing platform. Antimicrob. Agents Chemother. 2013, 57, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Jeffries, L.; Mach, K.E.; Craft, D.W.; Thomas, N.J.; Gau, V.; Liao, J.C.; Wong, P.K. A multiplex electrochemical biosensor for bloodstream infection diagnosis. SLAS Technol. 2017, 22, 466–474. [Google Scholar] [CrossRef]
- Altobelli, E.; Mohan, R.; Mach, K.E.; Sin, M.L.Y.; Anikst, V.; Buscarini, M.; Wong, P.K.; Gau, V.; Banaei, N.; Liao, J.C. Integrated biosensor assay for rapid uropathogen identification and phenotypic antimicrobial susceptibility testing. Eur. Urol. Focus 2017, 3, 293–299. [Google Scholar] [CrossRef]
- Ding, S.; Mosher, C.; Lee, X.Y.; Das, S.R.; Cargill, A.A.; Tang, X.; Chen, B.; McLamore, E.S.; Gomes, C.; Hostetter, J.M.; et al. Rapid and Label-free detection of interferon gamma via an electrochemical aptasensor comprising a ternary surface monolayer on a gold interdigitated electrode array. ACS Sens. 2017, 2, 210–217. [Google Scholar] [CrossRef]
- Dharuman, V.; Chang, B.-Y.; Park, S.-M.; Hahn, J.H. Ternary mixed monolayers for simultaneous DNA orientation control and surface passivation for label free DNA hybridization electrochemical sensing. Biosens. Bioelectron. 2010, 25, 2129–2134. [Google Scholar] [CrossRef]
- Dharuman, V.; Vijayaraj, K.; Radhakrishnan, S.; Dinakaran, T.; Narayanan, J.S.; Bhuvana, M.; Wilson, J. Sensitive label-free electrochemical DNA hybridization detection in the presence of 11-mercaptoundecanoic acid on the thiolated single strand DNA and mercaptohexanol binary mixed monolayer surface. Electrochim. Acta 2011, 56, 8147–8155. [Google Scholar] [CrossRef]
- González-Fernández, E.; Avlonitis, N.; Murray, A.F.; Mount, A.R.; Bradley, M. Methylene blue not ferrocene: Optimal reporters for electrochemical detection of protease activity. Biosens. Bioelectron. 2016, 84, 82–88. [Google Scholar] [CrossRef] [Green Version]
- González-Fernández, E.; Staderini, M.; Yussof, A.; Scholefield, E.; Murray, A.F.; Mount, A.R.; Bradley, M. Electrochemical sensing of human neutrophil elastase and polymorphonuclear neutrophil activity. Biosens. Bioelectron. 2018, 119, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Miodek, A.; Regan, E.M.; Bhalla, N.; Hopkins, N.A.E.; Goodchild, S.A.; Estrela, P. Optimisation and characterisation of anti-fouling ternary SAM layers for impedance-based aptasensors. Sensors 2015, 15, 25015–25032. [Google Scholar] [CrossRef] [PubMed]
- Henry, O.Y.F.; Acero Sanchez, J.L.; O’Sullivan, C.K. Bipodal PEGylated alkanethiol for the enhanced electrochemical detection of genetic markers involved in breast cancer. Biosens. Bioelectron. 2010, 26, 1500–1506. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.P.; Berry, R.M.; Turberfield, A.J. The single-step synthesis of a DNA tetrahedron. Chem. Commun. 2004, 12, 1372–1373. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; Lu, N.; Wen, Y.; Song, S.; Liu, Y.; Yan, H.; Fan, C. A DNA Nanostructure-based biomolecular probe carrier platform for electrochemical biosensing. Adv. Mater. 2010, 22, 4754–4758. [Google Scholar] [CrossRef]
- Wen, Y.; Pei, H.; Wan, Y.; Su, Y.; Huang, Q.; Song, S.; Fan, C. DNA nanostructure-decorated surfaces for enhanced aptamer-target binding and electrochemical cocaine sensors. Anal. Chem. 2011, 83, 7418–7423. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Lin, M.; Wang, P.; Pei, H.; Yan, J.; Shi, J.; Huang, Q.; He, D.; Fan, C.; Zuo, X. Hybridization chain reaction amplification of microRNA detection with a tetrahedral DNA nanostructure-based electrochemical biosensor. Anal. Chem. 2014, 86, 2124–2130. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, G.; Song, P.; Wang, J.; Gao, J.; Lu, J.; Fan, C.; Zuo, X. Ultrasensitive electrochemical detection of prostate-specific antigen by using antibodies anchored on a DNA nanostructural scaffold. Anal. Chem. 2014, 86, 7337–7342. [Google Scholar] [CrossRef]
- Lin, M.; Song, P.; Zhou, G.; Zuo, X.; Aldalbahi, A.; Lou, X.; Shi, J.; Fan, C. Electrochemical detection of nucleic acids, proteins, small molecules and cells using a DNA-nanostructure-based universal biosensing platform. Nat. Protoc. 2016, 11, 1244–1263. [Google Scholar] [CrossRef]
- Lin, M.; Wang, J.; Zhou, G.; Wang, J.; Wu, N.; Lu, J.; Gao, J.; Chen, X.; Shi, J.; Zuo, X.; et al. Programmable engineering of a biosensing interface with tetrahedral DNA nanostructures for ultrasensitive DNA detection. Angew. Chem. Int. Ed. 2015, 54, 2151–2155. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Zhao, R.; Zhu, J.; Lu, X.; Li, Y.; Qiu, S.; Ji, L.; Jiao, X.; Song, S.; Fan, C.; et al. Electrochemical DNA biosensor based on a tetrahedral nanostructure probe for the detection of Avian Influenza A (H7N9) virus. ACS Appl. Mater. Interfaces 2015, 5, 8834–8842. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Pei, H.; Wang, L.H.; Song, S.P.; Fan, C.H. Electrochemical single nucleotide polymorphisms genotyping on surface immobilized three-dimensional branched DNA nanostructure. Sci. China Chem. 2011, 54, 1273–1276. [Google Scholar] [CrossRef]
- Zeng, D.; Zhang, H.; Zhu, D.; Li, J.; San, L.; Wang, Z.; Wang, C.; Wang, Y.; Wang, L.; Zuo, X.; et al. A novel ultrasensitive electrochemical DNA sensor based on double tetrahedral nanostructures. Biosens. Bioelectron. 2015, 71, 434–4387. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; Wan, Y.; Li, J.; Hu, H.; Su, Y.; Huang, Q.; Fan, C. Regenerable electrochemical immunological sensing at DNA nanostructure-decorated gold surfaces. Chem. Commun. 2011, 47, 6254–6256. [Google Scholar] [CrossRef]
- Wang, J.; Leong, M.C.; Leong, E.Z.W.; Kuan, W.S.; Leong, D.T. Clinically relevant detection of Streptococcus pneumoniae with DNA-antibody nanostructures. Anal. Chem. 2017, 89, 6900–6906. [Google Scholar] [CrossRef]
- Miao, P.; Wang, B.; Meng, F.; Yin, J.; Tang, Y. Ultrasensitive detection of microRNA through rolling circle amplification on a DNA tetrahedron decorated electrode. Bioconjug. Chem. 2015, 26, 602–607. [Google Scholar] [CrossRef]
- Miao, P.; Tang, Y.; Yin, J. MicroRNA detection based on analyte triggered nanoparticle localization on a tetrahedral DNA modified electrode followed by hybridization chain reaction dual amplification. Chem. Commun. 2015, 51, 15629–15632. [Google Scholar] [CrossRef]
- Huang, Y.L.; Mo, S.; Gao, Z.F.; Chen, J.R.; Lei, J.L.; Luo, H.Q.; Li, N.B. Amperometric biosensor for microRNA based on the use of tetrahedral DNA nanostructure probes and guanine nanowire amplification. Microchim. Acta 2017, 184, 2597–2604. [Google Scholar] [CrossRef]
- Wen, Y.; Pei, H.; Shen, Y.; Xi, J.; Lin, M.; Lu, N.; Shen, X.; Li, J.; Fan, C. DNA Nanostructure-based interfacial engineering for PCR-free ultrasensitive electrochemical analysis of microRNA. Sci. Rep. 2012, 2, 867. [Google Scholar] [CrossRef]
- Wen, Y.; Liu, G.; Pei, H.; Li, L.; Xu, Q.; Liang, W.; Li, Y.; Xu, L.; Ren, S.; Fan, C. DNA nanostructure-based ultrasensitive electrochemical microRNA biosensor. Methods 2013, 64, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Wen, Y.; Li, L.; Pei, H.; Liu, G.; Song, H.; Zuo, X.; Fan, C.; Huang, Q. Target-responsive, DNA nanostructure-based E-DNA sensor for microRNA analysis. Anal. Chem. 2014, 86, 2285–2288. [Google Scholar] [CrossRef] [PubMed]
- McQuistan, A.; Zaitouna, A.J.; Echeverria, E.; Lai, R.Y. Use of thiolated oligonucleotides as anti-fouling diluents in electrochemical peptide-based sensors. Chem. Commun. 2014, 50, 4690–4692. [Google Scholar] [CrossRef] [PubMed]
- Jolly, P.; Formisano, N.; Tkáč, J.; Kasák, P.; Frost, C.G.; Estrela, P. Label-free impedimetric aptasensor with antifouling surface chemistry: A prostate specific antigen case study. Sens. Actuators B Chem. 2015, 209, 306–312. [Google Scholar] [CrossRef] [Green Version]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campuzano, S.; Pedrero, M.; Yáñez-Sedeño, P.; Pingarrón, J.M. Antifouling (Bio)materials for Electrochemical (Bio)sensing. Int. J. Mol. Sci. 2019, 20, 423. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20020423
Campuzano S, Pedrero M, Yáñez-Sedeño P, Pingarrón JM. Antifouling (Bio)materials for Electrochemical (Bio)sensing. International Journal of Molecular Sciences. 2019; 20(2):423. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20020423
Chicago/Turabian StyleCampuzano, Susana, María Pedrero, Paloma Yáñez-Sedeño, and José M. Pingarrón. 2019. "Antifouling (Bio)materials for Electrochemical (Bio)sensing" International Journal of Molecular Sciences 20, no. 2: 423. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20020423