Ganoderma lucidum Polysaccharides Prevent Palmitic Acid-Evoked Apoptosis and Autophagy in Intestinal Porcine Epithelial Cell Line via Restoration of Mitochondrial Function and Regulation of MAPK and AMPK/Akt/mTOR Signaling Pathway
Abstract
:1. Introduction
2. Results
2.1. GLP Suppressed PA-Mediated Cell Viability Loss in IPEC-J2 Cells
2.2. Effect of GLP on Cell Morphology in PA-Induced IPEC-J2 Cells
2.3. GLP Restored PA-Caused Loss of Mitochondrial Membrane Potential in IPEC-J2 Cells
2.4. GLP Alleviated the Increase of LDH Release in PA-Stimulated IPEC-J2 Cells
2.5. GLP Moderated PA-Triggered Apoptosis in IPEC-J2 Cells
2.6. GLP Modulated PA-Induced Energy Metabolism in IPEC-J2 Cells
2.7. GLP Inhibited PA-Induced Autophagy in IPEC-J2 Cells
2.8. GLP Decreased the Phosphorylation of MAPK Signaling Pathway in PA-Induced IPEC-J2 Cells
2.9. GLP Suppressed Akt/mTOR Pathway Inactivation Mediated by PA in IPEC-J2 Cells
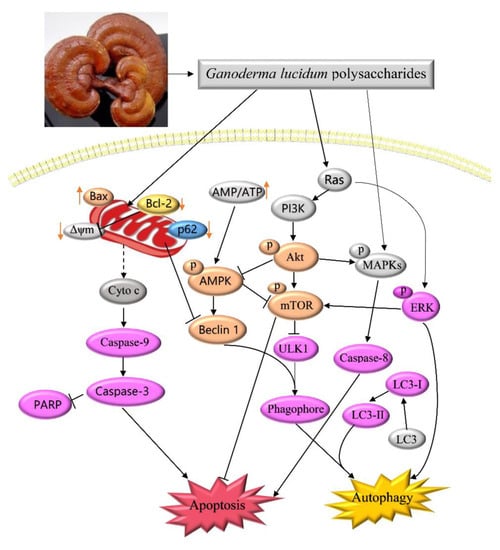
3. Discussion
4. Materials and Methods
4.1. Preparation of GLP
4.2. Cell Line and Culture
4.3. Assessment of Cell Viability by MTT
4.4. DAPI Staining
4.5. Detection of Mitochondrial Membrane Potential
4.6. Cell Membrane Integrity Assay
4.7. Apoptosis
4.8. Citrate Synthase Activity Assay
4.9. Determination of the Level of Mitochondrial ATP
4.10. Immunofluorescence
4.11. Western Blot Analysis
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Moreira, A.P.B.; Texeira, T.F.S.; Ferreira, A.B.; Peluzio, M.C.G.; Alfenas, R.C.G. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br. J. Nutr. 2012, 108, 801–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbari, P.; Fink-Gremmels, J.; Willems, R.H.A.M.; Difilippo, E.; Schols, M.H.C.; Garssen, J.; Braber, S. Characterizing microbiota-independent effects of oligosaccharides on intestinal epithelial cells: Insight into the role of structure and size. Eur. J. Nutr. 2017, 56, 1919–1930. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Dalmasso, G.; Müller, S.; Carrière, J.; Seibold, F.; Darfeuille-Michaud, A. Crohn’s disease-associated adherent invasive escherichia coli modulate levels of microRNAs in intestinal epithelial cells to reduce autophagy. Gastroenterology 2014, 146, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Elkahoui, S.; Bartley, G.E.; Yokoyama, W.H.; Friedman, M. Dietary supplementation of potato peel powders prepared from conventional and organic russet and non-organic gold and red potatoes reduces weight gain in mice on a high-fat diet. J. Agric. Food Chem. 2018, 66, 6064–6072. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Kim, S.; Guo, W.M.; Collins, F.W.; Wise, M.L.; Meydani, M. High levels of avenanthramides in oat-based diet further suppress high fat diet-induced atherosclerosis in Ldlr–/– mice. J. Agric. Food Chem. 2018, 66, 498–504. [Google Scholar] [CrossRef]
- Risio, M.; Lipkin, M.; Newmark, H.; Yang, K.; Rossini, F.P.; Steele, V.E.; Boone, C.W.; Kelloff, G.J. Apoptosis, cell replication, and western-style diet-induced tumorigenesis in mouse colon. Cancer Res. 1996, 56, 4910–4916. [Google Scholar]
- Luo, J.M.; Zhang, C.; Liu, R.; Gao, L.J.; Ou, S.Y.; Liu, L.; Peng, X.C. Ganoderma lucidum polysaccharide alleviating colorectal cancer by alteration of special gut bacteria and regulation of gene expression of colonic epithelial cells. J. Funct. Foods 2018, 47, 127–135. [Google Scholar] [CrossRef]
- Sun, X.Z.; Liao, Y.; Li, W.; Guo, L. Neuroprotective effects of Ganoderma lucidum polysaccharides against oxidative stress-induced neuronal apoptosis and antimicrobial activities. Neural Regen. Res. 2017, 12, 953–958. [Google Scholar]
- Xiao, C.; Wu, Q.; Zhang, J.; Xie, Y.Z.; Cai, W.; Tan, J.B. Antidiabetic activity of Ganoderma lucidum polysaccharides F31 downregulated hepatic glucose regulatory enzymes in diabetic mice. J. Ethnopharmacol. 2017, 196, 47–57. [Google Scholar] [CrossRef]
- Xiao, C.; Wu, Q.P.; Cai, W.; Tan, J.B.; Yang, X.B.; Zhang, J.M. Hypoglycemic effects of Ganoderma lucidum polysaccharides in type 2 diabetic mice. Arch. Pharm. Res. 2012, 35, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gorle, A.K.; Ainsworth, T.D.; Heimann, K.; Collins, J.G.; Keene, F.R. RNA and DNA binding of inert oligonuclear ruthenium (II) complexes in live eukaryotic cells. Dalton Trans. 2015, 44, 3594–3603. [Google Scholar] [CrossRef] [PubMed]
- Jaenisch, R.B.; Bertagnolli, M.; Borghi-Silva, A.; Arena, R.; Lago, P.D. Respiratory muscle training improves diaphragm citrate synthase activity and hemodynamic function in rats with heart failure. Braz. J. Cardiovasc. Surg. 2017, 32, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Das, D.N.; Panda, P.K.; Sinha, N.; Naik, P.P.; Bissoyi, A.; Pramanik, K.; Bhutia, S.K. Autophagy protein Ulk1 promotes mitochondrial apoptosis through reactive oxygen species. Free Radic. Biol. Med. 2015, 89, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, L.; Mnari, A.; Abdel-Daim, M.M.; Abid-Essafi, S.; Aleya, L. Therapeutic properties in Tunisian hot springs: First evidence of phenolic compounds in the cyanobacterium Leptolyngbya sp. biomass, capsular polysaccharides and releasing polysaccharides. BMC Complement. Altern. Med. 2016, 16, 515. [Google Scholar] [CrossRef] [PubMed]
- Meurens, F.; Summerfield, A.; Nauwynck, H.; Saif, L.; Gerdts, V. The pig: A model for human infectious diseases. Trends Microbiol. 2012, 20, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.K.C.; Ezzelarab, M.B.; Hara, H.; Iwase, H.; Lee, W.; Wijkstrom, M.; Bottino, R. The pathobiology of pig-to-primate xenotransplantation: A historical review. Xenotransplantation 2016, 23, 83–105. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Yuan, Z.H.; Li, G.Y.; Fu, F.H.; Shan, Y. Hypolipidemic, antioxidant, and antiapoptotic effects of polysaccharides extracted from reishi mushroom, Ganoderma lucidum (Leyss: Fr) Karst, in mice fed a high-fat die. J. Med. Food 2018. [Google Scholar] [CrossRef]
- Zhang, J.J.; Gao, X.; Pan, Y.G.; Xu, N.; Le, J. Toxicology and immunology of Ganoderma lucidum polysaccharides in Kunming mice and Wistar rats. Int. J. Biol. Macromol. 2016, 85, 302–310. [Google Scholar] [CrossRef]
- Wang, J.; Aung, L.H.H.; Prabhakar, B.S.; Li, P. The mitochondrial ubiquitin ligase plays an anti-apoptotic role in cardiomyocytes by regulating mitochondrial fission. J. Cell. Mol. Med. 2016, 20, 2278–2288. [Google Scholar] [CrossRef] [Green Version]
- Ke, F.F.S.; Vanyai, H.K.; Cowan, A.D.; Delbridge, A.R.D.; Whitehead, L.; Grabow, S.; Czabotar, P.E.; Voss, A.K.; Strasser, A. Embryogenesis and adult life in the absence of intrinsic apoptosis effectors BAX, BAK, and BOK. Cell 2018, 173, 1217–1230. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.J. Necrosis and apoptosis in the gastrointestinal tract. Gut 1995, 37, 165–167. [Google Scholar] [CrossRef]
- Dannappel, M.; Vlantis, K.; Kumari, S.; Polykratis, A.; Kim, C.; Wachsmuth, L.; Eftychi, C.; Lin, J.; Corona, T.; Hermance, N.; et al. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature 2014, 513, 90–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schüll, S.; Günther, S.D.; Brodesser, S.; Seeger, J.M.; Tosetti, B.; Wiegmann, K.; Pongratz, C.; Diaz, F.; Witt, A.; Andree, M.; et al. Cytochrome c oxidase deficiency accelerates mitochondrial apoptosis by activating ceramide synthase 6. Cell Death Dis. 2015, 6, e1691. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Cheng, T.L.; Tsai, W.H.; Tsai, H.J.; Hu, K.H.; Chang, H.C.; Yeh, C.W.; Chen, Y.C.; Liao, C.C.; Chang, W.T. Loss of the respiratory enzyme citrate synthase directly links the Warburg effect to tumor malignancy. Sci. Rep. 2012, 2, 785. [Google Scholar] [CrossRef]
- Cai, Q.X.; Zhao, M.M.; Liu, X.; Wang, X.C.; Nie, Y.; Li, P.; Liu, T.; Ge, R.; Han, F.C. Reduced expression of citrate synthase leads to excessive superoxide formation and cell apoptosis. Biochem. Biophys. Res. Commun. 2017, 485, 388–394. [Google Scholar] [CrossRef]
- Jaiswal, N.; Maurya, C.K.; Arha, D.; Avisetti, D.R.; Prathapan, A.; Raj, P.S.; Raghu, K.G.; Kalivendi, S.V.; Tamrakar, A.K. Fructose induces mitochondrial dysfunction and triggers apoptosis in skeletal muscle cells by provoking oxidative stress. Apoptosis 2015, 20, 930–947. [Google Scholar] [CrossRef]
- Dagon, Y.; Avraham, Y.; Berry, E.M. AMPK activation regulates apoptosis, adipogenesis, and lipolysis by eIF2α in adipocytes. Biochem. Biophys. Res. Commun. 2006, 340, 43–47. [Google Scholar] [CrossRef]
- D’Assantea, R.; Fuscoa, A.; Palamaro, L.; Polishchuk, E.; Polishchuk, R.; Bianchino, G.; Grieco, V.; Prencipe, M.R.; Ballabio, A.; Pignata, C. Abnormal cell-clearance and accumulation of autophagic vesicles in lymphocytes from patients affected with Ataxia-Teleangiectasia. Clin. Immunol. 2017, 175, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Jiang, P.; Mizushima, N. LC3- and p62-based biochemical methods for the analysis of autophagy progression in mammalian cells. Methods 2015, 75, 13–18. [Google Scholar] [CrossRef]
- Fernández, F.; Sebti, S.; Wei, Y.; Zou, Z.; Shi, M.; McMillan, K.L.; Schiattarella, G.G.; Bhagat, G.; Moe, O.W.; Hu, M.C.; et al. Disruption of the beclin 1–BCL2 autophagy regulatory complex promotes longevity in mice. Nature 2018, 561, 136–140. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, H.F.; Ren, H.G.; Chen, D.; Gao, F.; Hu, Q.S.; Fu, C.; Xu, R.J.; Ying, Z.; Wang, G.H. Bcl-2-dependent upregulation of autophagy by sequestosome 1/p62 in vitro. Acta Pharmacol. Sin. 2013, 34, 651–656. [Google Scholar] [CrossRef]
- Komatsu, M.; Waguri, S.; Koike, M.; Sou, Y.S.; Ueno, T.; Hara, T.; Mizushima, T.; Iwata, J.; Ezaki, J.; Murata, J.; et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 2007, 131, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.M.J.; Csibi, A.; Raibon, A.; Cornille, K.; Gay, S.; Bernardi, H.; Candau, R. AMPK promotes skeletal muscle autophagy through activation of forkhead FoxO3a and interaction with Ulk1. J. Cell. Biochem. 2012, 113, 695–710. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Choi, E.J. Compromised MAPK signaling in human diseases: An update. Arch. Toxicol. 2015, 89, 867–882. [Google Scholar] [CrossRef]
- Sui, X.B.; Kong, N.; Ye, L.; Han, W.D.; Zhou, J.C.; Zhang, Q.; He, C.; Pan, H.M. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett. 2014, 344, 174–179. [Google Scholar] [CrossRef]
- Shen, Y.; Yang, J.J.; Zhao, J.; Xiao, C.J.; Xu, C.M.; Xiang, Y. The switch from ER stress-induced apoptosis to autophagy via ROS-mediated JNK/p62 signals: A survival mechanism in methotrexate-resistant choriocarcinoma cells. Exp. Cell Res. 2015, 334, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Junttila, M.R.; Li, S.P.; Westermarck, J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J. 2007, 22, 954–965. [Google Scholar] [CrossRef]
- Kim, K.Y.; Park, K.; Kim, S.H.; Yu, S.N.; Park, S.G.; Kim, Y.W.; Seo, Y.K.; Ma, J.Y.; Ahn, S.C. Inhibition of autophagy promotes salinomycin-induced apoptosis via reactive oxygen species-mediated PI3K/AKT/mTOR and ERK/p38 MAPK-dependent signaling in human prostate cancer cells. Int. J. Mol. Sci. 2017, 18, 1088. [Google Scholar] [CrossRef]
- Liu, J.; Chang, F.; Li, F.; Fu, H.; Wang, J.; Zhang, S.; Zhao, J.; Yin, D. Palmitate promotes autophagy and apoptosis through ROS-dependent JNK and p38 MAPK. Biochem. Biophys. Res. Commun. 2015, 463, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.D.; Li, Y.; Zheng, H.Y.; Tong, Y.Q.; Dai, W. Palmitate induces H9c2 cell apoptosis by increasing reactive oxygen species generation and activation of the ERK1/2 signaling pathway. Mol. Med. Rep. 2013, 7, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Wang, J.; Liu, J.; Xia, M.; Li, W.; He, M. Macrophage immigration inhibitory factor promotes cell proliferation and inhibits apoptosis of cervical adenocarcinoma. Tumor Biol. 2015, 36, 5095–5102. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, C.; Chen, S.; Ye, Y.; Guo, M.; Ren, Q.; Liu, L.; Zhang, H.; Xu, C.; Zhou, Q.; et al. Activation of AMPK and inactivation of Akt result in suppression of mTOR-mediated S6K1 and 4E-BP1 pathways leading to neuronal cell death in in vitro models of Parkinson’s disease. Cell. Signal. 2014, 26, 1680–1689. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Das, B.; Sen, R.; Kundu, P.; Manna, A.; Sarkar, A.; Chowdhury, C.; Chatterjee, M.; Das, P. Andrographolide analogue induces apoptosis and autophagy mediated cell death in U937 cells by inhibition of PI3K/Akt/mTOR pathway. PLoS ONE 2015, 10, e0139657. [Google Scholar] [CrossRef]
- Asnaghi, L.; Calastretti, A.; Bevilacqua, A.; D’Agnano, I.; Gatti, G.; Canti, G.; Delia, D.; Capaccioli, S.; Nicolin, A. Bcl-2 phosphorylation and apoptosis activated by damaged microtubules require mTOR and are regulated by Akt. Oncogene 2004, 23, 5781–5791. [Google Scholar] [CrossRef] [Green Version]
- Pan, B.S.; Wang, Y.K.; Lai, M.S.; Mu, Y.F.; Huang, B.M. Cordycepin induced MA-10 mouse Leydig tumor cell apoptosis by regulating p38 MAPKs and PI3K/AKT signaling pathways. Sci. Rep. 2015, 5, 13372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Z.; Yi, Y.J.; Guo, Y.T.; Wang, R.C.; Hu, Q.L.; Xiong, X.Y. Chemical characterization and antitumor activities of polysaccharide extracted from Ganoderma lucidum. Int. J. Mol. Sci. 2014, 15, 9103–9116. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Li, Q.; Huang, Q.; Li, X.; Yin, M.; Wang, Z.; Hong, J. Cardioprotective effect of propofol against oxygen glucose deprivation and reperfusion injury in H9c2 cells. Oxidative Med. Cell. Longev. 2017, 2015, 184938. [Google Scholar]
- Rhoads, J.M.; Chen, W.; Chu, P.; Berschneider, H.M.; Argenzio, R.A.; Paradiso, A.M. L-Glutamine and L-asparagine stimulate Na+–H+ exchange in porcine jejunal enterocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 1994, 266, 828–838. [Google Scholar] [CrossRef]
- Wang, J.; Hu, G.D.; Lin, Z.; He, L.; Xu, L.; Zhang, Y.M. Characteristic and functional analysis of a newly established porcine small intestinal epithelial cell line. PLoS ONE 2014, 9, e110916. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Z.; Yuan, Z.; Guo, J.; Wu, J.; Yi, J.; Deng, J.; Shan, Y. Ganoderma lucidum Polysaccharides Prevent Palmitic Acid-Evoked Apoptosis and Autophagy in Intestinal Porcine Epithelial Cell Line via Restoration of Mitochondrial Function and Regulation of MAPK and AMPK/Akt/mTOR Signaling Pathway. Int. J. Mol. Sci. 2019, 20, 478. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20030478
Liang Z, Yuan Z, Guo J, Wu J, Yi J, Deng J, Shan Y. Ganoderma lucidum Polysaccharides Prevent Palmitic Acid-Evoked Apoptosis and Autophagy in Intestinal Porcine Epithelial Cell Line via Restoration of Mitochondrial Function and Regulation of MAPK and AMPK/Akt/mTOR Signaling Pathway. International Journal of Molecular Sciences. 2019; 20(3):478. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20030478
Chicago/Turabian StyleLiang, Zengenni, Zhihang Yuan, Jiajing Guo, Jing Wu, Jine Yi, Jing Deng, and Yang Shan. 2019. "Ganoderma lucidum Polysaccharides Prevent Palmitic Acid-Evoked Apoptosis and Autophagy in Intestinal Porcine Epithelial Cell Line via Restoration of Mitochondrial Function and Regulation of MAPK and AMPK/Akt/mTOR Signaling Pathway" International Journal of Molecular Sciences 20, no. 3: 478. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20030478