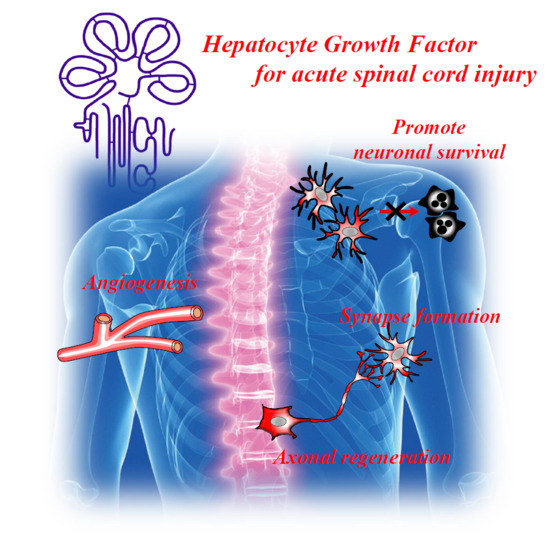
Application of Hepatocyte Growth Factor for Acute Spinal Cord Injury: The Road from Basic Studies to Human Treatment
Abstract
:1. Introduction
1.1. Time Course of the Endogenous Hepatocyte Growth Factor–c-Met System after Spinal Cord Injury in Rats: Endogenous Hepatocyte Growth Factor Upregulation is Weak in the Spinal Cord during the Acute Injury Phase
1.2. Introducing Exogenous Hepatocyte Growth Factor into the Spinal Cord using the Herpes Simplex Virus-1 Vector Exerted Multiple Therapeutic Effects to Promote Functional Recovery in a Rat Thoracic Spinal Cord Injury Model: Neuroprotection and Promotion of Angiogenic Activity and Axonal Regrowth
1.3. Other Recent Basic Studies on Hepatocyte Growth Factor Treatment for Spinal Cord Injury
1.3.1. Reduction of Astrocyte Activation and Chondroitin Sulfate Proteoglycan Deposition
1.3.2. Anti-Inflammatory Effect and Reduction in Leukocyte Infiltration
1.3.3. Increasing the Survival, Neuronal Differentiation, and Synapse Formation of Grafted Neural Stem Cells to Promote Functional Recovery
1.4. Summary of the Therapeutic Mechanisms of Hepatocyte Growth Factor in Rodents
2. Steps Toward Starting a Clinical Trial
2.1. Translation from Gene Therapy to the Intrathecal Administration of Recombinant Human Hepatocyte Growth Factor, and from Rat Thoracic Spinal Cord Injury to Primate Cervical Spinal Cord Injury
2.1.1. Advantages of Using a Cervical SCI Model in Marmosets for Preclinical Trials
2.1.2. Intrathecal Recombinant Human Hepatocyte Growth Factor Promotes Neurological Hand Function and Reduces the Damaged Area in a Non-Human Primate after Spinal Cord Injury
2.1.3. Therapeutic Efficacy of Intrathecally Administered Recombinant Human Hepatocyte Growth Factor in a Severe Cervical Spinal Cord Injury Marmoset Model
2.2. Therapeutic Time Window and Minimal Effective Dose of Intrathecal Recombinant Human Hepatocyte Growth Factor
2.2.1. Delayed Intrathecal Recombinant Human Hepatocyte Growth Factor startinga 4 Days after SCI Improved the Functional Recovery after Spinal Cord Injury in Rats
2.2.2. Minimal Effective Dose of Intrathecal rhHGF
2.2.3. Advantages and Disadvantages of Intrathecal Administration
3. Phase I/II Clinical Trial for Acute Cervical SCI in Japan
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jain, N.B.; Ayers, G.D.; Peterson, E.N.; Harris, M.B.; Morse, L.; O’Connor, K.C.; Garshick, E. Traumatic spinal cord injury in the United States, 1993–2012. JAMA 2015, 313, 2236–2243. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, L.H.; Fehlings, M.G. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine 2001, 26, S2–S12. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.H.; Wuermser, L.A.; Priebe, M.M.; Chiodo, A.E.; Scelza, W.M.; Kirshblum, S.C. Spinal cord injury medicine. 1. Epidemiology and classification. Arch. Phys. Med. Rehabil. 2007, 88, S49–S54. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Ha, K.Y.; Kim, S.I. Spinal Cord Injury and Related Clinical Trials. Clin. Orthop. Surg. 2017, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pickett, G.E.; Campos-Benitez, M.; Keller, J.L.; Duggal, N. Epidemiology of traumatic spinal cord injury in Canada. Spine 2006, 31, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, M.E.; Castellote, J.M.; Mahillo-Fernandez, I.; de Pedro-Cuesta, J. Incidence of spinal cord injury worldwide: A systematic review. Neuroepidemiology 2010, 34, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Oyinbo, C.A. Secondary injury mechanisms in traumatic spinal cord injury: A nugget of this multiply cascade. Acta Neurobiol. Exp. (Wars) 2011, 71, 281–299. [Google Scholar] [PubMed]
- Witiw, C.D.; Fehlings, M.G. Acute Spinal Cord Injury. J. Spinal Disord. Tech. 2015, 28, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Bracken, M.B.; Shepard, M.J.; Collins, W.F.; Holford, T.R.; Young, W.; Baskin, D.S.; Eisenberg, H.M.; Flamm, E.; Leo-Summers, L.; Maroon, J.; et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N. Engl. J. Med. 1990, 322, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.G.; Wilson, J.R.; Cho, N. Methylprednisolone for the treatment of acute spinal cord injury: Counterpoint. Neurosurgery 2014, 61 (Suppl. 1), 36–42. [Google Scholar] [CrossRef] [PubMed]
- Hurlbert, R.J. Methylprednisolone for the treatment of acute spinal cord injury: Point. Neurosurgery 2014, 61 (Suppl. 1), 32–35. [Google Scholar] [CrossRef] [PubMed]
- Hurlbert, R.J.; Hadley, M.N.; Walters, B.C.; Aarabi, B.; Dhall, S.S.; Gelb, D.E.; Rozzelle, C.J.; Ryken, T.C.; Theodore, N. Pharmacological therapy for acute spinal cord injury. Neurosurgery 2013, 72 (Suppl. 2), 93–105. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Tamaki, T.; Kawakami, M.; Yoshida, M.; Ando, M.; Yamada, H. Early complications of high-dose methylprednisolone sodium succinate treatment in the follow-up of acute cervical spinal cord injury. Spine 2001, 26, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Nawa, K.; Ichihara, A. Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem. Biophys. Res. Commun. 1984, 122, 1450–1459. [Google Scholar] [CrossRef]
- Miyazawa, K.; Tsubouchi, H.; Naka, D.; Takahashi, K.; Okigaki, M.; Arakaki, N.; Nakayama, H.; Hirono, S.; Sakiyama, O.; et al. Molecular cloning and sequence analysis of cDNA for human hepatocyte growth factor. Biochem. Biophys. Res. Commun. 1989, 163, 967–973. [Google Scholar] [CrossRef]
- Nakamura, T.; Nishizawa, T.; Hagiya, M.; Seki, T.; Shimonishi, M.; Sugimura, A.; Tashiro, K.; Shimizu, S. Molecular cloning and expression of human hepatocyte growth factor. Nature 1989, 342, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Bottaro, D.P.; Rubin, J.S.; Faletto, D.L.; Chan, A.M.; Kmiecik, T.E.; Vande Woude, G.F.; Aaronson, S.A. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science 1991, 251, 802–804. [Google Scholar] [CrossRef] [PubMed]
- Kato, T. Biological roles of hepatocyte growth factor-Met signaling from genetically modified animals. Biomed. Rep. 2017, 7, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Funakoshi, H.; Takahashi, H.; Sakai, K. HGF-Met Pathway in Regeneration and Drug Discovery. Biomedicines 2014, 2, 275–300. [Google Scholar] [CrossRef] [PubMed]
- Date, I.; Takagi, N.; Takagi, K.; Kago, T.; Matsumoto, K.; Nakamura, T.; Takeo, S. Hepatocyte growth factor attenuates cerebral ischemia-induced learning dysfunction. Biochem. Biophys. Res. Commun. 2004, 319, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Date, I.; Takagi, N.; Takagi, K.; Kago, T.; Matsumoto, K.; Nakamura, T.; Takeo, S. Hepatocyte growth factor improved learning and memory dysfunction of microsphere-embolized rats. J. Neurosci. Res. 2004, 78, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Deguchi, K.; Ohta, Y.; Liu, N.; Zhang, X.; Tian, F.; Yamashita, T.; Ikeda, Y.; Matsuura, T.; Funakoshi, H.; et al. Strong neurogenesis, angiogenesis, synaptogenesis, and antifibrosis of hepatocyte growth factor in rats brain after transient middle cerebral artery occlusion. J. Neurosci. Res. 2011, 89, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Matsumoto, K.; Ohmichi, H.; Katoh, H.; Yamashima, T.; Nakamura, T. Protection of hippocampal neurons from ischemia-induced delayed neuronal death by hepatocyte growth factor: A novel neurotrophic factor. J. Cereb. Blood Flow Metab. 1998, 18, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, M.; Sato, N.; Oshima, K.; Aoki, M.; Kurinami, H.; Waguri, S.; Uchiyama, Y.; Ogihara, T.; Kaneda, Y.; Morishita, R. Novel therapeutic strategy to treat brain ischemia: Overexpression of hepatocyte growth factor gene reduced ischemic injury without cerebral edema in rat model. Circulation 2004, 109, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, M.; Sato, N.; Waguri, S.; Uchiyama, Y.; Hayashi, T.; Iida, H.; Nakamura, T.; Ogihara, T.; Kaneda, Y.; Morishita, R. Gene transfer of hepatocyte growth factor gene improves learning and memory in the chronic stage of cerebral infarction. Hypertension 2006, 47, 742–751. [Google Scholar] [CrossRef] [PubMed]
- Niimura, M.; Takagi, N.; Takagi, K.; Funakoshi, H.; Nakamura, T.; Takeo, S. Effects of hepatocyte growth factor on phosphorylation of extracellular signal-regulated kinase and hippocampal cell death in rats with transient forebrain ischemia. Eur. J. Pharmacol. 2006, 535, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Niimura, M.; Takagi, N.; Takagi, K.; Mizutani, R.; Ishihara, N.; Matsumoto, K.; Funakoshi, H.; Nakamura, T.; Takeo, S. Prevention of apoptosis-inducing factor translocation is a possible mechanism for protective effects of hepatocyte growth factor against neuronal cell death in the hippocampus after transient forebrain ischemia. J. Cereb. Blood Flow Metab. 2006, 26, 1354–1365. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Funakoshi, H.; Nakamura, T. Overexpression of HGF retards disease progression and prolongs life span in a transgenic mouse model of ALS. J. Neurosci. 2002, 22, 6537–6548. [Google Scholar] [CrossRef] [PubMed]
- Ishigaki, A.; Aoki, M.; Nagai, M.; Warita, H.; Kato, S.; Kato, M.; Nakamura, T.; Funakoshi, H.; Itoyama, Y. Intrathecal delivery of hepatocyte growth factor from amyotrophic lateral sclerosis onset suppresses disease progression in rat amyotrophic lateral sclerosis model. J. Neuropathol. Exp. Neurol. 2007, 66, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Warita, H.; Kato, M.; Asada, R.; Yamashita, A.; Hayata, D.; Adachi, K.; Aoki, M. Safety, Tolerability, and Pharmacodynamics of Intrathecal Injection of Recombinant Human HGF (KP-100) in Subjects With Amyotrophic Lateral Sclerosis: A Phase I Trial. J. Clin. Pharmacol. 2018. Available online: https://0-accp1-onlinelibrary-wiley-com.brum.beds.ac.uk/doi/abs/10.1002/jcph.1355 (accessed on 11 December 2018). [CrossRef]
- Kitamura, K.; Iwanami, A.; Nakamura, M.; Yamane, J.; Watanabe, K.; Suzuki, Y.; Miyazawa, D.; Shibata, S.; Funakoshi, H.; Miyatake, S.; et al. Hepatocyte growth factor promotes endogenous repair and functional recovery after spinal cord injury. J. Neurosci. Res. 2007, 85, 2332–2342. [Google Scholar] [CrossRef] [PubMed]
- Kono, S.; Nagaike, M.; Matsumoto, K.; Nakamura, T. Marked induction of hepatocyte growth factor mRNA in intact kidney and spleen in response to injury of distant organs. Biochem. Biophys. Res. Commun. 1992, 186, 991–998. [Google Scholar] [CrossRef]
- Matsumoto, K.; Nakamura, T. Hepatocyte growth factor (HGF) as a tissue organizer for organogenesis and regeneration. Biochem. Biophys. Res. Commun. 1997, 239, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Noji, S.; Tashiro, K.; Koyama, E.; Nohno, T.; Ohyama, K.; Taniguchi, S.; Nakamura, T. Expression of hepatocyte growth factor gene in endothelial and Kupffer cells of damaged rat livers, as revealed by in situ hybridization. Biochem. Biophys. Res. Commun. 1990, 173, 42–47. [Google Scholar] [CrossRef]
- Yanagita, K.; Matsumoto, K.; Sekiguchi, K.; Ishibashi, H.; Niho, Y.; Nakamura, T. Hepatocyte growth factor may act as a pulmotrophic factor on lung regeneration after acute lung injury. J. Biol. Chem. 1993, 268, 21212–21217. [Google Scholar] [PubMed]
- Igawa, T.; Matsumoto, K.; Kanda, S.; Saito, Y.; Nakamura, T. Hepatocyte growth factor may function as a renotropic factor for regeneration in rats with acute renal injury. Am. J. Physiol. 1993, 265, F61–F69. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, T.; Nagayama, M.; Kohara, S.; Kamiguchi, H.; Shibuya, M.; Katoh, Y.; Itoh, J.; Shinohara, Y. Post-ischemic delayed expression of hepatocyte growth factor and c-Met in mouse brain following focal cerebral ischemia. Brain Res. 2004, 999, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Caton, A.; Hacker, A.; Naeem, A.; Livet, J.; Maina, F.; Bladt, F.; Klein, R.; Birchmeier, C.; Guthrie, S. The branchial arches and HGF are growth-promoting and chemoattractant for cranial motor axons. Development 2000, 127, 1751–1766. [Google Scholar] [PubMed]
- Maina, F.; Klein, R. Hepatocyte growth factor, a versatile signal for developing neurons. Nat. Neurosci. 1999, 2, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Hamanoue, M.; Takemoto, N.; Matsumoto, K.; Nakamura, T.; Nakajima, K.; Kohsaka, S. Neurotrophic effect of hepatocyte growth factor on central nervous system neurons in vitro. J. Neurosci. Res. 1996, 43, 554–564. [Google Scholar] [CrossRef]
- Okura, Y.; Arimoto, H.; Tanuma, N.; Matsumoto, K.; Nakamura, T.; Yamashima, T.; Miyazawa, T.; Matsumoto, Y. Analysis of neurotrophic effects of hepatocyte growth factor in the adult hypoglossal nerve axotomy model. Eur. J. Neurosci. 1999, 11, 4139–4144. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Morishita, R.; Nakagami, H.; Yoshimura, S.; Hara, A.; Matsumoto, K.; Nakamura, T.; Ogihara, T.; Kaneda, Y.; Sakai, N. Gene therapy for preventing neuronal death using hepatocyte growth factor: In vivo gene transfer of HGF to subarachnoid space prevents delayed neuronal death in gerbil hippocampal CA1 neurons. Gene Ther. 2001, 8, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Morishita, R.; Higaki, J.; Kida, I.; Aoki, M.; Moriguchi, A.; Yamada, K.; Hayashi, S.; Yo, Y.; Matsumoto, K.; et al. Expression of local hepatocyte growth factor system in vascular tissues. Biochem. Biophys. Res. Commun. 1995, 215, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Morishita, R.; Nakamura, S.; Aoki, M.; Moriguchi, A.; Matsumoto, K.; Nakamura, T.; Higaki, J.; Ogihara, T. A vascular modulator, hepatocyte growth factor, is associated with systolic pressure. Hypertension 1996, 28, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Morishita, R.; Aoki, M.; Hashiya, N.; Yamasaki, K.; Kurinami, H.; Shimizu, S.; Makino, H.; Takesya, Y.; Azuma, J.; Ogihara, T. Therapeutic angiogenesis using hepatocyte growth factor (HGF). Curr. Gene Ther. 2004, 4, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Loy, D.N.; Crawford, C.H.; Darnall, J.B.; Burke, D.A.; Onifer, S.M.; Whittemore, S.R. Temporal progression of angiogenesis and basal lamina deposition after contusive spinal cord injury in the adult rat. J. Comp. Neurol. 2002, 445, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Hagg, T.; Oudega, M. Degenerative and spontaneous regenerative processes after spinal cord injury. J. Neurotrauma 2006, 23, 264–280. [Google Scholar] [CrossRef] [PubMed]
- Beattie, M.S.; Bresnahan, J.C.; Komon, J.; Tovar, C.A.; Van Meter, M.; Anderson, D.K.; Faden, A.I.; Hsu, C.Y.; Noble, L.J.; Salzman, S.; et al. Endogenous repair after spinal cord contusion injuries in the rat. Exp. Neurol. 1997, 148, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Casella, G.T.; Marcillo, A.; Bunge, M.B.; Wood, P.M. New vascular tissue rapidly replaces neural parenchyma and vessels destroyed by a contusion injury to the rat spinal cord. Exp. Neurol. 2002, 173, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Guizar-Sahagun, G.; Ibarra, A.; Espitia, A.; Martinez, A.; Madrazo, I.; Franco-Bourland, R.E. Glutathione monoethyl ester improves functional recovery, enhances neuron survival, and stabilizes spinal cord blood flow after spinal cord injury in rats. Neuroscience 2005, 130, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Kawabe, J.; Koda, M.; Hashimoto, M.; Fujiyoshi, T.; Furuya, T.; Endo, T.; Okawa, A.; Yamazaki, M. Neuroprotective effects of granulocyte colony-stimulating factor and relationship to promotion of angiogenesis after spinal cord injury in rats: Laboratory investigation. J. Neurosurg. Spine 2011, 15, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Ebens, A.; Brose, K.; Leonardo, E.D.; Hanson, M.G., Jr.; Bladt, F.; Birchmeier, C.; Barres, B.A.; Tessier-Lavigne, M. Hepatocyte growth factor/scatter factor is an axonal chemoattractant and a neurotrophic factor for spinal motor neurons. Neuron 1996, 17, 1157–1172. [Google Scholar] [CrossRef]
- Wong, V.; Glass, D.J.; Arriaga, R.; Yancopoulos, G.D.; Lindsay, R.M.; Conn, G. Hepatocyte growth factor promotes motor neuron survival and synergizes with ciliary neurotrophic factor. J. Biol. Chem. 1997, 272, 5187–5191. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, T.; Muroya, K.; Mukasa, T.; Igarashi, H.; Momoi, M.; Tsukahara, T.; Arahata, K.; Kumagai, H.; Momoi, T. Hepatocyte growth factor specifically expressed in microglia activated Ras in the neurons, similar to the action of neurotrophic factors. Biochem. Biophys. Res. Commun. 1995, 210, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Bregman, B.S. Spinal cord transplants permit the growth of serotonergic axons across the site of neonatal spinal cord transection. Brain Res. 1987, 431, 265–279. [Google Scholar] [CrossRef]
- Kim, J.E.; Liu, B.P.; Park, J.H.; Strittmatter, S.M. Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron 2004, 44, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Saruhashi, Y.; Young, W.; Perkins, R. The recovery of 5-HT immunoreactivity in lumbosacral spinal cord and locomotor function after thoracic hemisection. Exp. Neurol. 1996, 139, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, T.; Nakamura, M.; Yamane, J.; Katoh, H.; Okada, S.; Iwanami, A.; Watanabe, K.; Ishii, K.; Kato, F.; Fujita, H.; et al. Chondroitinase ABC combined with neural stem/progenitor cell transplantation enhances graft cell migration and outgrowth of growth-associated protein-43-positive fibers after rat spinal cord injury. Eur. J. Neurosci. 2005, 22, 3036–3046. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.R.; Fan, D.P.; Giehl, K.M.; Bedard, A.M.; Wiegand, S.J.; Tetzlaff, W. BDNF and NT-4/5 prevent atrophy of rat rubrospinal neurons after cervical axotomy, stimulate GAP-43 and Talpha1-tubulin mRNA expression, and promote axonal regeneration. J. Neurosci. 1997, 17, 9583–9595. [Google Scholar] [CrossRef] [PubMed]
- Ramon-Cueto, A.; Plant, G.W.; Avila, J.; Bunge, M.B. Long-distance axonal regeneration in the transected adult rat spinal cord is promoted by olfactory ensheathing glia transplants. J. Neurosci. 1998, 18, 3803–3815. [Google Scholar] [CrossRef] [PubMed]
- Morgenstern, D.A.; Asher, R.A.; Fawcett, J.W. Chondroitin sulphate proteoglycans in the CNS injury response. Prog. Brain Res. 2002, 137, 313–332. [Google Scholar] [PubMed]
- Silver, J.; Miller, J.H. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004, 5, 146–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, S.R.; Kwon, M.J.; Lee, H.G.; Joe, E.H.; Lee, J.H.; Kim, S.S.; Suh-Kim, H.; Kim, B.G. Hepatocyte growth factor reduces astrocytic scar formation and promotes axonal growth beyond glial scars after spinal cord injury. Exp. Neurol. 2012, 233, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Giannopoulou, M.; Dai, C.; Tan, X.; Wen, X.; Michalopoulos, G.K.; Liu, Y. Hepatocyte growth factor exerts its anti-inflammatory action by disrupting nuclear factor-kappaB signaling. Am. J. Pathol. 2008, 173, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Gong, R. Multi-target anti-inflammatory action of hepatocyte growth factor. Curr. Opin. Investig. Drugs 2008, 9, 1163–1170. [Google Scholar] [PubMed]
- Yamane, K.; Mazaki, T.; Shiozaki, Y.; Yoshida, A.; Shinohara, K.; Nakamura, M.; Yoshida, Y.; Zhou, D.; Kitajima, T.; Tanaka, M.; et al. Collagen-Binding Hepatocyte Growth Factor (HGF) alone or with a Gelatin- furfurylamine Hydrogel Enhances Functional Recovery in Mice after Spinal Cord Injury. Sci. Rep. 2018, 8, 917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takano, M.; Kawabata, S.; Shibata, S.; Yasuda, A.; Nori, S.; Tsuji, O.; Nagoshi, N.; Iwanami, A.; Ebise, H.; Horiuchi, K.; et al. Enhanced Functional Recovery from Spinal Cord Injury in Aged Mice after Stem Cell Transplantation through HGF Induction. Stem Cell Rep. 2017, 8, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Kokuzawa, J.; Yoshimura, S.; Kitajima, H.; Shinoda, J.; Kaku, Y.; Iwama, T.; Morishita, R.; Shimazaki, T.; Okano, H.; Kunisada, T.; et al. Hepatocyte growth factor promotes proliferation and neuronal differentiation of neural stem cells from mouse embryos. Mol. Cell. Neurosci. 2003, 24, 190–197. [Google Scholar] [CrossRef]
- Kato, M.; Yoshimura, S.; Kokuzawa, J.; Kitajima, H.; Kaku, Y.; Iwama, T.; Shinoda, J.; Kunisada, T.; Sakai, N. Hepatocyte growth factor promotes neuronal differentiation of neural stem cells derived from embryonic stem cells. Neuroreport 2004, 15, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, K.; Fujiyoshi, K.; Yamane, J.; Toyota, F.; Hikishima, K.; Nomura, T.; Funakoshi, H.; Nakamura, T.; Aoki, M.; Toyama, Y.; et al. Human hepatocyte growth factor promotes functional recovery in primates after spinal cord injury. PLoS ONE 2011, 6, e27706. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, K.; Iwanami, A.; Iwai, H.; Toyama, Y.; Matsumoto, M.; Okano, H.; Nakamura, M. Therapeutic time window and preclinical efficacy of intrathecal administration of recombinant human hepatocyte growth factor for acute spinal cord injury. J. Spine Res. 2016, 7, 934–939. [Google Scholar]
- Lemon, R. Cortico-motoneuronal system and dexterous finger movements. J. Neurophysiol. 2004, 92, 3601–3603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamane, J.; Nakamura, M.; Iwanami, A.; Sakaguchi, M.; Katoh, H.; Yamada, M.; Momoshima, S.; Miyao, S.; Ishii, K.; Tamaoki, N.; et al. Transplantation of galectin-1-expressing human neural stem cells into the injured spinal cord of adult common marmosets. J. Neurosci. Res. 2010, 88, 1394–1405. [Google Scholar] [CrossRef] [PubMed]
- Iwanami, A.; Yamane, J.; Katoh, H.; Nakamura, M.; Momoshima, S.; Ishii, H.; Tanioka, Y.; Tamaoki, N.; Nomura, T.; Toyama, Y.; et al. Establishment of graded spinal cord injury model in a nonhuman primate: The common marmoset. J. Neurosci. Res. 2005, 80, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Iwanami, A.; Kaneko, S.; Nakamura, M.; Kanemura, Y.; Mori, H.; Kobayashi, S.; Yamasaki, M.; Momoshima, S.; Ishii, H.; Ando, K.; et al. Transplantation of human neural stem cells for spinal cord injury in primates. J. Neurosci. Res. 2005, 80, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Fujiyoshi, K.; Yamada, M.; Nakamura, M.; Yamane, J.; Katoh, H.; Kitamura, K.; Kawai, K.; Okada, S.; Momoshima, S.; Toyama, Y.; et al. In vivo tracing of neural tracts in the intact and injured spinal cord of marmosets by diffusion tensor tractography. J. Neurosci. 2007, 27, 11991–11998. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.; Sasaki, T.; Shimizu, A.; Yoshida, K.; Iwai, H.; Koya, I.; Kobayashi, Y.; Itakura, G.; Shibata, S.; Ebise, H.; et al. Global gene expression analysis following spinal cord injury in non-human primates. Exp. Neurol. 2014, 261, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Inada, T.; Takahashi, H.; Yamazaki, M.; Okawa, A.; Sakuma, T.; Kato, K.; Hashimoto, M.; Hayashi, K.; Furuya, T.; Fujiyoshi, T.; et al. Multicenter prospective nonrandomized controlled clinical trial to prove neurotherapeutic effects of granulocyte colony-stimulating factor for acute spinal cord injury: Analyses of follow-up cases after at least 1 year. Spine 2014, 39, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Koda, M.; Furuya, T.; Kato, K.; Takahashi, H.; Sakuma, T.; Inada, T.; Ota, M.; Maki, S.; Okawa, A.; et al. Neuroprotective therapy with granulocyte colony-stimulating factor in acute spinal cord injury: A comparison with high-dose methylprednisolone as a historical control. Eur. Spine J. 2015, 24, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Koda, M.; Hanaoka, H.; Sato, T.; Fujii, Y.; Hanawa, M.; Takahashi, S.; Furuya, T.; Ijima, Y.; Saito, J.; Kitamura, M.; et al. Study protocol for the G-SPIRIT trial: A randomised, placebo-controlled, double-blinded phase III trial of granulocyte colony-stimulating factor-mediated neuroprotection for acute spinal cord injury. BMJ Open 2018, 8, e019083. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.G.; Nakashima, H.; Nagoshi, N.; Chow, D.S.; Grossman, R.G.; Kopjar, B. Rationale, design and critical end points for the Riluzole in Acute Spinal Cord Injury Study (RISCIS): A randomized, double-blinded, placebo-controlled parallel multi-center trial. Spinal Cord. 2016, 54, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.G.; Wilson, J.R.; Frankowski, R.F.; Toups, E.G.; Aarabi, B.; Harrop, J.S.; Shaffrey, C.I.; Harkema, S.J.; Guest, J.D.; Tator, C.H.; et al. Riluzole for the treatment of acute traumatic spinal cord injury: Rationale for and design of the NACTN Phase I clinical trial. J. Neurosurg. Spine 2012, 17, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.G.; Theodore, N.; Harrop, J.; Maurais, G.; Kuntz, C.; Shaffrey, C.I.; Kwon, B.K.; Chapman, J.; Yee, A.; Tighe, A.; et al. A phase I/IIa clinical trial of a recombinant Rho protein antagonist in acute spinal cord injury. J. Neurotrauma 2011, 28, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Kopp, M.A.; Liebscher, T.; Watzlawick, R.; Martus, P.; Laufer, S.; Blex, C.; Schindler, R.; Jungehulsing, G.J.; Knuppel, S.; Kreutztrager, M.; et al. SCISSOR-Spinal Cord Injury Study on Small molecule-derived Rho inhibition: A clinical study protocol. BMJ Open 2016, 6, e010651. [Google Scholar] [CrossRef] [PubMed]
- Casha, S.; Zygun, D.; McGowan, M.D.; Bains, I.; Yong, V.W.; Hurlbert, R.J. Results of a phase II placebo-controlled randomized trial of minocycline in acute spinal cord injury. Brain 2012, 135, 1224–1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aghazadeh, J.; Samadi Motlagh, P.; Salehpour, F.; Meshkini, A.; Fatehi, M.; Mirzaei, F.; Naseri Alavi, S.A. Effects of Atorvastatin in Patients with Acute Spinal Cord Injury. Asian Spine J. 2017, 11, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Yu, Y.; Yemane, R.; Cain, C.; Yu, C.; Kastin, A.J. Permeation of hepatocyte growth factor across the blood-brain barrier. Exp. Neurol. 2006, 201, 99–104. [Google Scholar] [CrossRef] [PubMed]

| Epithelial Organs (Liver, Lung, or Kidney) |  | Spinal Cord |
|---|---|---|
| Marked increase within 24 h after injury | Endogenous upregulation of HGF in the injured organ | Weak and delayed, with a peak around 4 weeks after injury |
| Marked increase within 24 h after injury | Delivery of HGF from other intact organs via an endocrine mechanism | No delivery |
| Reference | SCI Model | Therapeutic Intervention | Timing of the Intervention | Therapeutic Effects |
|---|---|---|---|---|
| Kitamura et al. (2007) [31] | Contusive thoracic SCI in adult rats | HSV-1 vector injection into spinal cord | 3 days prior to SCI | Promoted the survival of neurons and oligodendrocytes, angiogenesis, and the axonal regrowth of 5-HT-positive fibers |
| Jeong et al. (2012) [63] | Hemisectional cervical SCI in adult rats | Transplantation of HGF-overexpressing MSCs into hemisectional lesion | Immediately after SCI | Diminished the TGF isoform levels, reduced astrocyte activation, and decreased the CSPG deposition around the lesion site to increase axonal growth beyond the glial scar. |
| Yamane et al. (2018) [66] | Compressive thoracic SCI in adult mice | Single intrathecal injection of engineered CBD-HGF | Immediately after SCI | CBD-HGF remained in the spinal cord for 7 days and exerted an anti-inflammatory effect by disrupting NF-κB signaling, decreasing cytokine levels, and reducing the infiltration of leukocytes and glial scar formation. |
| Takano et al. (2017) [67] | Contusive thoracic SCI in aged and young mice | Transplantation of NSCs | 9 days after SCI | HGF was the most highly expressed neurotrophic factor in aged mice compared to young ones at the time of transplantation, and promoted the survival, neuronal differentiation, and synapse formation of grafted NSCs. |
| Kitamura et al. (2011) [70] | Contusive cervical SCI in adult common marmosets | Intrathecal infusion of 400 µg of rhHGF for 4 weeks | Starting immediately after SCI | Preserved myelinated white matter and the CST pathway and promoted hand function |
| Kitamura et al. (2016) [71] | Contusive thoracic SCI in adult rats | Intrathecal infusion of rhHGF (1) 200 µg for 2 weeks, starting immediately after SCI; (2) 8, 40, or 200 µg for 2 weeks starting 4 days after SCI; (3) 400 µg for 4 weeks, starting 2 or 8 weeks after SCI | Promoted functional recovery when intrathecal infusion was started immediately after or 4 days after SCI | |
| More severe contusive cervical SCI than in [69] in adult common marmosets | Intrathecal infusion of 400 µg of rhHGF for 4 weeks | Starting immediately after SCI | All marmosets showed no recovery in upper limb motor function until 4 days after SCI. At least one key muscle in upper limb became useful in rhHGF-treated animals, whereas all the key muscles remained useless in the control animals. | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitamura, K.; Nagoshi, N.; Tsuji, O.; Matsumoto, M.; Okano, H.; Nakamura, M. Application of Hepatocyte Growth Factor for Acute Spinal Cord Injury: The Road from Basic Studies to Human Treatment. Int. J. Mol. Sci. 2019, 20, 1054. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20051054
Kitamura K, Nagoshi N, Tsuji O, Matsumoto M, Okano H, Nakamura M. Application of Hepatocyte Growth Factor for Acute Spinal Cord Injury: The Road from Basic Studies to Human Treatment. International Journal of Molecular Sciences. 2019; 20(5):1054. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20051054
Chicago/Turabian StyleKitamura, Kazuya, Narihito Nagoshi, Osahiko Tsuji, Morio Matsumoto, Hideyuki Okano, and Masaya Nakamura. 2019. "Application of Hepatocyte Growth Factor for Acute Spinal Cord Injury: The Road from Basic Studies to Human Treatment" International Journal of Molecular Sciences 20, no. 5: 1054. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20051054