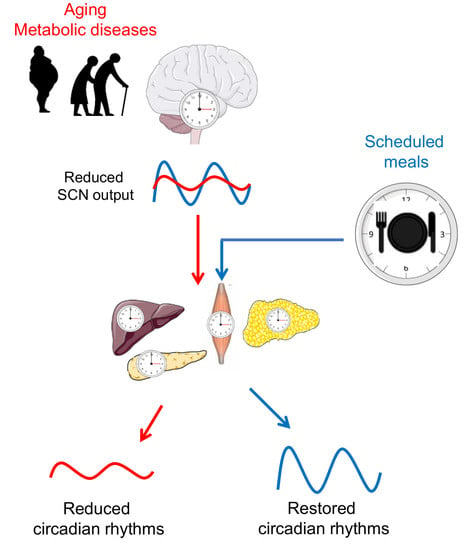
Meal Timing, Aging, and Metabolic Health
Abstract
:1. Introduction
2. Circadian Regulation of Metabolism
3. Circadian Rhythms in Later Life
4. Timing of Eating as an Important Factor of Metabolic Regulation
5. Time-Restricted Feeding as a Promising Tool for Circadian and Metabolic Improvements
6. Other Dietary Approaches Affecting the Circadian Clock
7. Conclusions
Funding
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| CR | Calorie restriction |
| FEO | Food-entrainable oscillator |
| HFD | High-fat diet |
| IF | Intermittent fasting |
| T2D | Type 2 diabetes |
| TRF | Time-restricted feeding |
References
- Panda, S. Circadian physiology of metabolism. Science 2016, 354, 1008–1015. [Google Scholar] [CrossRef]
- Antunes, L.C.; Levandovski, R.; Dantas, G.; Caumo, W.; Hidalgo, M.P. Obesity and shift work: Chronobiological aspects. Nutr. Res. Rev. 2010, 23, 155–168. [Google Scholar] [CrossRef] [PubMed]
- De Bacquer, D.; Van Risseghem, M.; Clays, E.; Kittel, F.; De Backer, G.; Braeckman, L. Rotating shift work and the metabolic syndrome: A prospective study. Int. J. Epidemiol. 2009, 38, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Vetter, C.; Devore, E.E.; Wegrzyn, L.R.; Massa, J.; Speizer, F.E.; Kawachi, I.; Rosner, B.; Stampfer, M.J.; Schernhammer, E.S. Association Between Rotating Night Shift Work and Risk of Coronary Heart Disease Among Women. JAMA J. Am. Med. Assoc. 2016, 315, 1726–1734. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Takamura, T.; Matsuzawa-Nagata, N.; Shima, K.R.; Eto, T.; Misu, H.; Shiramoto, M.; Tsuru, T.; Irie, S.; Fujimura, A.; et al. Clock gene expression in peripheral leucocytes of patients with type 2 diabetes. Diabetologia 2009, 52, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Vieira, E.; Ruano, E.; Figueroa, A.L.; Aranda, G.; Momblan, D.; Carmona, F.; Gomis, R.; Vidal, J.; Hanzu, F.A. Altered clock gene expression in obese visceral adipose tissue is associated with metabolic syndrome. PloS ONE 2014, 9, e111678. [Google Scholar] [CrossRef]
- Gomez-Abellan, P.; Hernandez-Morante, J.J.; Lujan, J.A.; Madrid, J.A.; Garaulet, M. Clock genes are implicated in the human metabolic syndrome. Int. J. Obes. 2008, 32, 121–128. [Google Scholar] [CrossRef]
- Hood, S.; Amir, S. The aging clock: Circadian rhythms and later life. J. Clin. Investig. 2017, 127, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Niccoli, T.; Partridge, L. Ageing as a risk factor for disease. Curr. Biol. 2012, 22, R741–R752. [Google Scholar] [CrossRef]
- Jiang, P.; Turek, F.W. Timing of meals: When is as critical as what and how much. Am. J. Physiol. Endocrinol. Metab. 2017, 312, E369–E380. [Google Scholar] [CrossRef]
- Asher, G.; Sassone-Corsi, P. Time for food: The intimate interplay between nutrition, metabolism, and the circadian clock. Cell 2015, 161, 84–92. [Google Scholar] [CrossRef]
- Allison, K.C.; Goel, N. Timing of eating in adults across the weight spectrum: Metabolic factors and potential circadian mechanisms. Physiol. Behav. 2018, 192, 158–166. [Google Scholar] [CrossRef]
- Johnston, J.D.; Ordovas, J.M.; Scheer, F.A.; Turek, F.W. Circadian Rhythms, Metabolism, and Chrononutrition in Rodents and Humans. Adv. Nutr. 2016, 7, 399–406. [Google Scholar] [CrossRef]
- Garrido, M.; Terron, M.P.; Rodriguez, A.B. Chrononutrition against oxidative stress in aging. Oxidative Med. Cell. Longev. 2013, 2013, 729804. [Google Scholar] [CrossRef]
- Brown, S.A. Circadian Metabolism: From Mechanisms to Metabolomics and Medicine. Trends Endocrinol. Metab. 2016, 27, 415–426. [Google Scholar] [CrossRef]
- Keller, M.; Mazuch, J.; Abraham, U.; Eom, G.D.; Herzog, E.D.; Volk, H.D.; Kramer, A.; Maier, B. A circadian clock in macrophages controls inflammatory immune responses. Proc. Natl. Acad. Sci. USA 2009, 106, 21407–21412. [Google Scholar] [CrossRef] [Green Version]
- Eckel-Mahan, K.L.; Patel, V.R.; de Mateo, S.; Orozco-Solis, R.; Ceglia, N.J.; Sahar, S.; Dilag-Penilla, S.A.; Dyar, K.A.; Baldi, P.; Sassone-Corsi, P. Reprogramming of the circadian clock by nutritional challenge. Cell 2013, 155, 1464–1478. [Google Scholar] [CrossRef]
- Wang, J.; Mauvoisin, D.; Martin, E.; Atger, F.; Galindo, A.N.; Dayon, L.; Sizzano, F.; Palini, A.; Kussmann, M.; Waridel, P.; et al. Nuclear Proteomics Uncovers Diurnal Regulatory Landscapes in Mouse Liver. Cell Metab. 2017, 25, 102–117. [Google Scholar] [CrossRef] [Green Version]
- Turek, F.W. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 2005, 308, 1043–1045. [Google Scholar] [CrossRef]
- Paschos, G.K.; Ibrahim, S.; Song, W.L.; Kunieda, T.; Grant, G.; Reyes, T.M.; Bradfield, C.A.; Vaughan, C.H.; Eiden, M.; Masoodi, M.; et al. Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat. Med. 2012, 18, 1768–1777. [Google Scholar] [CrossRef] [Green Version]
- Lamia, K.A.; Storch, K.F.; Weitz, C.J. Physiological significance of a peripheral tissue circadian clock. Proc. Natl. Acad. Sci. USA 2008, 105, 15172–15177. [Google Scholar] [CrossRef] [Green Version]
- Chaix, A.; Zarrinpar, A.; Miu, P.; Panda, S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014, 20, 991–1005. [Google Scholar] [CrossRef] [Green Version]
- Kohsaka, A.; Laposky, A.D.; Ramsey, K.M.; Estrada, C.; Joshu, C.; Kobayashi, Y.; Turek, F.W.; Bass, J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007, 6, 414–421. [Google Scholar] [CrossRef]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.; et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef]
- Su, W.; Xie, Z.; Guo, Z.; Duncan, M.J.; Lutshumba, J.; Gong, M.C. Altered clock gene expression and vascular smooth muscle diurnal contractile variations in type 2 diabetic db/db mice. Am. J. Physiol. 2012, 302, H621–H633. [Google Scholar] [CrossRef]
- Ando, H.; Kumazaki, M.; Motosugi, Y.; Ushijima, K.; Maekawa, T.; Ishikawa, E.; Fujimura, A. Impairment of peripheral circadian clocks precedes metabolic abnormalities in ob/ob mice. Endocrinology 2011, 152, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Pivovarova, O.; Jurchott, K.; Rudovich, N.; Hornemann, S.; Ye, L.; Mockel, S.; Murahovschi, V.; Kessler, K.; Seltmann, A.C.; Maser-Gluth, C.; et al. Changes of Dietary Fat and Carbohydrate Content Alter Central and Peripheral Clock in Humans. J. Clin. Endocrinol. Metab. 2015, 100, 2291–2302. [Google Scholar] [CrossRef] [Green Version]
- Chaudhari, A.; Gupta, R.; Makwana, K.; Kondratov, R. Circadian clocks, diets and aging. Nutr. Healthy Aging 2017, 4, 101–112. [Google Scholar] [CrossRef] [Green Version]
- Davidson, A.J. Chronic jet-lag increases mortality in aged mice. Curr. Biol. 2006, 16, R914–R916. [Google Scholar] [CrossRef]
- Yu, E.A.; Weaver, D.R. Disrupting the circadian clock: Gene-specific effects on aging, cancer, and other phenotypes. Aging 2011, 3, 479–493. [Google Scholar] [CrossRef] [Green Version]
- Froy, O. Circadian rhythms, aging, and life span in mammals. Physiology 2011, 26, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Froy, O.; Chapnik, N.; Miskin, R. Long-lived alphaMUPA transgenic mice exhibit pronounced circadian rhythms. Am. J. Physiol. 2006, 291, E1017–E1024. [Google Scholar]
- Fonseca Costa, S.S.; Ripperger, J.A. Impact of the circadian clock on the aging process. Front. Neurol. 2015, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Roenneberg, T.; Kuehnle, T.; Juda, M.; Kantermann, T.; Allebrandt, K.; Gordijn, M.; Merrow, M. Epidemiology of the human circadian clock. Sleep Med. Rev. 2007, 11, 429–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, C.; Peigneux, P.; Cajochen, C.; Collette, F. Adapting test timing to the sleep-wake schedule: Effects on diurnal neurobehavioral performance changes in young evening and older morning chronotypes. Chronobiol. Int. 2012, 29, 482–490. [Google Scholar] [CrossRef]
- Banks, G.; Nolan, P.M.; Peirson, S.N. Reciprocal interactions between circadian clocks and aging. Mamm. Genome 2016, 27, 332–340. [Google Scholar] [CrossRef] [Green Version]
- Dijk, D.J.; Duffy, J.F.; Czeisler, C.A. Contribution of circadian physiology and sleep homeostasis to age-related changes in human sleep. Chronobiol. Int. 2000, 17, 285–311. [Google Scholar] [CrossRef]
- Hayashi, Y.; Endo, S. All-night sleep polygraphic recordings of healthy aged persons: REM and slow-wave sleep. Sleep 1982, 5, 277–283. [Google Scholar] [CrossRef]
- Huang, Y.L.; Liu, R.Y.; Wang, Q.S.; Van Someren, E.J.; Xu, H.; Zhou, J.N. Age-associated difference in circadian sleep-wake and rest-activity rhythms. Physiol. Behav. 2002, 76, 597–603. [Google Scholar] [CrossRef]
- Touitou, Y.; Fevre, M.; Lagoguey, M.; Carayon, A.; Bogdan, A.; Reinberg, A.; Beck, H.; Cesselin, F.; Touitou, C. Age- and mental health-related circadian rhythms of plasma levels of melatonin, prolactin, luteinizing hormone and follicle-stimulating hormone in man. J. Endocrinol. 1981, 91, 467–475. [Google Scholar] [CrossRef]
- Van Cauter, E.; Leproult, R.; Kupfer, D.J. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J. Clin. Endocrinol. Metab. 1996, 81, 2468–2473. [Google Scholar] [PubMed]
- Wijsman, C.A.; van Heemst, D.; Hoogeveen, E.S.; Slagboom, P.E.; Maier, A.B.; de Craen, A.J.; van der Ouderaa, F.; Pijl, H.; Westendorp, R.G.; Mooijaart, S.P. Ambulant 24-h glucose rhythms mark calendar and biological age in apparently healthy individuals. Aging Cell 2013, 12, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Singh, R.K.; Masood, T.; Tripathi, A.K.; Mahdi, A.A.; Singh, R.K.; Schwartzkopff, O.; Cornelissen, G. Circadian time structure of circulating plasma lipid peroxides, antioxidant enzymes and other small molecules in peptic ulcers. Clin. Chim. Acta 2015, 451, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Mazzoccoli, G.; Inglese, M.; De Cata, A.; Carughi, S.; Dagostino, M.P.; Marzulli, N.; Damato, M.; Grilli, M.; Giuliani, F.; Greco, A. Neuroendocrine-immune interactions in healthy aging. Geriatr. Gerontol. Int. 2011, 11, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Deleidi, M.; Jaggle, M.; Rubino, G. Immune aging, dysmetabolism, and inflammation in neurological diseases. Front. Neurosci. 2015, 9, 172. [Google Scholar] [CrossRef]
- Chen, C.Y.; Logan, R.W.; Ma, T.; Lewis, D.A.; Tseng, G.C.; Sibille, E.; McClung, C.A. Effects of aging on circadian patterns of gene expression in the human prefrontal cortex. Proc. Natl. Acad. Sci. USA 2016, 113, 206–211. [Google Scholar] [CrossRef]
- Bonaconsa, M.; Malpeli, G.; Montaruli, A.; Carandente, F.; Grassi-Zucconi, G.; Bentivoglio, M. Differential modulation of clock gene expression in the suprachiasmatic nucleus, liver and heart of aged mice. Exp. Gerontol. 2014, 55, 70–79. [Google Scholar] [CrossRef]
- Kolker, D.E.; Fukuyama, H.; Huang, D.S.; Takahashi, J.S.; Horton, T.H.; Turek, F.W. Aging alters circadian and light-induced expression of clock genes in golden hamsters. J. Biol. Rhythm. 2003, 18, 159–169. [Google Scholar] [CrossRef]
- Yamazaki, S.; Straume, M.; Tei, H.; Sakaki, Y.; Menaker, M.; Block, G.D. Effects of aging on central and peripheral mammalian clocks. Proc. Natl. Acad. Sci. USA 2002, 99, 10801–10806. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, T.J.; Nakamura, W.; Yamazaki, S.; Kudo, T.; Cutler, T.; Colwell, C.S.; Block, G.D. Age-related decline in circadian output. J. Neurosci. 2011, 31, 10201–10205. [Google Scholar] [CrossRef]
- Luo, W.; Chen, W.F.; Yue, Z.; Chen, D.; Sowcik, M.; Sehgal, A.; Zheng, X. Old flies have a robust central oscillator but weaker behavioral rhythms that can be improved by genetic and environmental manipulations. Aging Cell 2012, 11, 428–438. [Google Scholar] [CrossRef] [Green Version]
- Sutton, G.M.; Ptitsyn, A.A.; Floyd, Z.E.; Yu, G.; Wu, X.; Hamel, K.; Shah, F.S.; Centanni, A.; Eilertsen, K.; Kheterpal, I.; et al. Biological aging alters circadian mechanisms in murine adipose tissue depots. Age 2013, 35, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Monk, T.H.; Buysse, D.J.; Carrier, J.; Kupfer, D.J. Inducing jet-lag in older people: Directional asymmetry. J. Sleep Res. 2000, 9, 101–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofman, M.A.; Swaab, D.F. Alterations in circadian rhythmicity of the vasopressin-producing neurons of the human suprachiasmatic nucleus (SCN) with aging. Brain Res. 1994, 651, 134–142. [Google Scholar] [CrossRef]
- Palomba, M.; Nygard, M.; Florenzano, F.; Bertini, G.; Kristensson, K.; Bentivoglio, M. Decline of the presynaptic network, including GABAergic terminals, in the aging suprachiasmatic nucleus of the mouse. J. Biol. Rhythm. 2008, 23, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Sellix, M.T.; Evans, J.A.; Leise, T.L.; Castanon-Cervantes, O.; Hill, D.D.; DeLisser, P.; Block, G.D.; Menaker, M.; Davidson, A.J. Aging differentially affects the re-entrainment response of central and peripheral circadian oscillators. J. Neurosci. 2012, 32, 16193–16202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walcott, E.C.; Tate, B.A. Entrainment of aged, dysrhythmic rats to a restricted feeding schedule. Physiol. Behav. 1996, 60, 1205–1208. [Google Scholar] [CrossRef]
- Izumo, M.; Pejchal, M.; Schook, A.C.; Lange, R.P.; Walisser, J.A.; Sato, T.R.; Wang, X.; Bradfield, C.A.; Takahashi, J.S. Differential effects of light and feeding on circadian organization of peripheral clocks in a forebrain Bmal1 mutant. Elife 2014, 3, 23. [Google Scholar] [CrossRef]
- Juarez, C.; Morgado, E.; Waliszewski, S.M.; Martinez, A.J.; Meza, E.; Caba, M. Synchronization of PER1 protein in parabrachial nucleus in a natural model of food anticipatory activity. Eur. J. Neurosci. 2012, 35, 1458–1465. [Google Scholar] [CrossRef]
- Yamanaka, A.; Tsunematsu, T. New approaches for the study of orexin function. J. Neuroendocrinol. 2010, 22, 818–824. [Google Scholar] [CrossRef]
- Mieda, M.; Williams, S.C.; Richardson, J.A.; Tanaka, K.; Yanagisawa, M. The dorsomedial hypothalamic nucleus as a putative food-entrainable circadian pacemaker. Proc. Natl. Acad. Sci. USA 2006, 103, 12150–12155. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, Y.; Kurasawa, M.; Nakamura, K. Recovery of diminished mealtime-associated anticipatory behavior by aniracetam in aged rats. Pharmacol. Biochem. Behav. 2000, 66, 827–833. [Google Scholar] [CrossRef]
- Damiola, F. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000, 14, 2950–2961. [Google Scholar] [CrossRef] [Green Version]
- Yasumoto, Y.; Hashimoto, C.; Nakao, R.; Yamazaki, H.; Hiroyama, H.; Nemoto, T.; Yamamoto, S.; Sakurai, M.; Oike, H.; Wada, N.; et al. Short-term feeding at the wrong time is sufficient to desynchronize peripheral clocks and induce obesity with hyperphagia, physical inactivity and metabolic disorders in mice. Metab. Clin. Exp. 2016, 65, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Arble, D.M.; Bass, J.; Laposky, A.D.; Vitaterna, M.H.; Turek, F.W. Circadian timing of food intake contributes to weight gain. Obesity 2009, 17, 2100–2102. [Google Scholar] [CrossRef] [PubMed]
- Fonken, L.K.; Workman, J.L.; Walton, J.C.; Weil, Z.M.; Morris, J.S.; Haim, A.; Nelson, R.J. Light at night increases body mass by shifting the time of food intake. Proc. Natl. Acad. Sci. USA 2010, 107, 18664–18669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karatsoreos, I.N.; Bhagat, S.; Bloss, E.B.; Morrison, J.H.; McEwen, B.S. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 1657–1662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salgado-Delgado, R.; Angeles-Castellanos, M.; Saderi, N.; Buijs, R.M.; Escobar, C. Food intake during the normal activity phase prevents obesity and circadian desynchrony in a rat model of night work. Endocrinology 2010, 151, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Scheer, F.A.; Hilton, M.F.; Mantzoros, C.S.; Shea, S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA 2009, 106, 4453–4458. [Google Scholar] [CrossRef] [Green Version]
- Archer, S.N.; Laing, E.E.; Moller-Levet, C.S.; van der Veen, D.R.; Bucca, G.; Lazar, A.S.; Santhi, N.; Slak, A.; Kabiljo, R.; von Schantz, M.; et al. Mistimed sleep disrupts circadian regulation of the human transcriptome. Proc. Natl. Acad. Sci. USA 2014, 111, E682–E691. [Google Scholar] [CrossRef] [Green Version]
- Wefers, J.; van Moorsel, D.; Hansen, J.; Connell, N.J.; Havekes, B.; Hoeks, J.; van Marken Lichtenbelt, W.D.; Duez, H.; Phielix, E.; Kalsbeek, A.; et al. Circadian misalignment induces fatty acid metabolism gene profiles and compromises insulin sensitivity in human skeletal muscle. Proc. Natl. Acad. Sci. USA 2018, 115, 7789–7794. [Google Scholar] [CrossRef]
- Garaulet, M.; Gomez-Abellan, P.; Alburquerque-Bejar, J.J.; Lee, Y.C.; Ordovas, J.M.; Scheer, F.A. Timing of food intake predicts weight loss effectiveness. Int. J. Obes. 2013, 37, 604–611. [Google Scholar] [CrossRef] [Green Version]
- Jakubowicz, D.; Barnea, M.; Wainstein, J.; Froy, O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity 2013, 21, 2504–2512. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, S.K.; Tang, T.S. When′s dinner? Does timing of dinner affect the cardiometabolic risk profiles of South-Asian Canadians at risk for diabetes. Diabet. Med. 2017, 34, 539–542. [Google Scholar] [CrossRef]
- Aljuraiban, G.S.; Chan, Q.; Oude Griep, L.M.; Brown, I.J.; Daviglus, M.L.; Stamler, J.; Van Horn, L.; Elliott, P.; Frost, G.S.; Group, I.R. The impact of eating frequency and time of intake on nutrient quality and Body Mass Index: The INTERMAP Study, a Population-Based Study. J. Acad. Nutr. Diet. 2015, 115, 528–536. [Google Scholar] [CrossRef]
- Gallant, A.R.; Lundgren, J.; Drapeau, V. The night-eating syndrome and obesity. Obes. Rev. 2012, 13, 528–536. [Google Scholar] [CrossRef]
- Wehrens, S.M.T.; Christou, S.; Isherwood, C.; Middleton, B.; Gibbs, M.A.; Archer, S.N.; Skene, D.J.; Johnston, J.D. Meal Timing Regulates the Human Circadian System. Curr. Biol. 2017, 27, 1768–1775. [Google Scholar] [CrossRef]
- LeCheminant, J.D.; Christenson, E.; Bailey, B.W.; Tucker, L.A. Restricting night-time eating reduces daily energy intake in healthy young men: A short-term cross-over study. Br. J. Nutr. 2013, 110, 2108–2113. [Google Scholar] [CrossRef]
- Hibi, M.; Masumoto, A.; Naito, Y.; Kiuchi, K.; Yoshimoto, Y.; Matsumoto, M.; Katashima, M.; Oka, J.; Ikemoto, S. Nighttime snacking reduces whole body fat oxidation and increases LDL cholesterol in healthy young women. Am. J. Physiol. 2013, 304, R94–R101. [Google Scholar] [CrossRef] [Green Version]
- Bandin, C.; Scheer, F.A.; Luque, A.J.; Avila-Gandia, V.; Zamora, S.; Madrid, J.A.; Gomez-Abellan, P.; Garaulet, M. Meal timing affects glucose tolerance, substrate oxidation and circadian-related variables: A randomized, crossover trial. Int. J. Obes. 2015, 39, 828–833. [Google Scholar] [CrossRef]
- Qin, L.Q.; Li, J.; Wang, Y.; Wang, J.; Xu, J.Y.; Kaneko, T. The effects of nocturnal life on endocrine circadian patterns in healthy adults. Life Sci. 2003, 73, 2467–2475. [Google Scholar] [CrossRef]
- McHill, A.W.; Phillips, A.J.; Czeisler, C.A.; Keating, L.; Yee, K.; Barger, L.K.; Garaulet, M.; Scheer, F.A.; Klerman, E.B. Later circadian timing of food intake is associated with increased body fat. Am. J. Clin. Nutr. 2017, 106, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Bray, M.S. Time-of-day-dependent dietary fat consumption influences multiple cardiometabolic syndrome parameters in mice. Int. J. Obes. 2014, 34, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- Kessler, K.; Hornemann, S.; Petzke, K.J.; Kemper, M.; Kramer, A.; Pfeiffer, A.F.; Pivovarova, O.; Rudovich, N. The effect of diurnal distribution of carbohydrates and fat on glycaemic control in humans: A randomized controlled trial. Sci. Rep. 2017, 7, 44170. [Google Scholar] [CrossRef]
- Almoosawi, S.; Prynne, C.J.; Hardy, R.; Stephen, A.M. Diurnal eating rhythms: Association with long-term development of diabetes in the 1946 British birth cohort. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Almoosawi, S.; Prynne, C.J.; Hardy, R.; Stephen, A.M. Time-of-day and nutrient composition of eating occasions: Prospective association with the metabolic syndrome in the 1946 British birth cohort. Int. J. Obes. 2013, 37, 725–731. [Google Scholar] [CrossRef]
- Kessler, K.; Hornemann, S.; Petzke, K.J.; Kemper, M.; Markova, M.; Rudovich, N.; Grune, T.; Kramer, A.; Pfeiffer, A.F.H.; Pivovarova-Ramich, O. Diurnal distribution of carbohydrates and fat affects substrate oxidation and adipokine secretion in humans. Am. J. Clin. Nutr 2018, 108, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Fadda, M.; Castiglione, A.; Ciccone, G.; De Francesco, A.; Fedele, D.; Guggino, A.; Parasiliti Caprino, M.; Ferrara, S.; Vezio Boggio, M.; et al. Is the timing of caloric intake associated with variation in diet-induced thermogenesis and in the metabolic pattern? A randomized cross-over study. Int. J. Obes. 2015, 39, 1689–1695. [Google Scholar] [CrossRef] [Green Version]
- Jakubowicz, D.; Wainstein, J.; Ahren, B.; Bar-Dayan, Y.; Landau, Z.; Rabinovitz, H.R.; Froy, O. High-energy breakfast with low-energy dinner decreases overall daily hyperglycaemia in type 2 diabetic patients: A randomised clinical trial. Diabetologia 2015, 58, 912–919. [Google Scholar] [CrossRef]
- Lindgren, O.; Mari, A.; Deacon, C.F.; Carr, R.D.; Winzell, M.S.; Vikman, J.; Ahren, B. Differential islet and incretin hormone responses in morning versus afternoon after standardized meal in healthy men. J. Clin. Endocrinol. Metab. 2009, 94, 2887–2892. [Google Scholar] [CrossRef]
- Sato, M.; Murakami, M.; Node, K.; Matsumura, R.; Akashi, M. The role of the endocrine system in feeding-induced tissue-specific circadian entrainment. Cell Rep. 2014, 8, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Landgraf, D.; Tsang, A.H.; Leliavski, A.; Koch, C.E.; Barclay, J.L.; Drucker, D.J.; Oster, H. Oxyntomodulin regulates resetting of the liver circadian clock by food. Elife 2015, 4, e06253. [Google Scholar] [CrossRef] [PubMed]
- Mukherji, A.; Kobiita, A.; Chambon, P. Shifting the feeding of mice to the rest phase creates metabolic alterations, which, on their own, shift the peripheral circadian clocks by 12 h. Proc. Natl. Acad. Sci. USA 2015, 112, E6683–E6690. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Panda, S. A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metab. 2015, 22, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Roenneberg, T.; Allebrandt, K.V.; Merrow, M.; Vetter, C. Social jetlag and obesity. Curr. Biol. 2012, 22, 939–943. [Google Scholar] [CrossRef]
- Gupta, N.J.; Kumar, V.; Panda, S. A camera-phone based study reveals erratic eating pattern and disrupted daily eating-fasting cycle among adults in India. PloS ONE 2017, 12, e0172852. [Google Scholar] [CrossRef] [PubMed]
- Anton, S.D.; Moehl, K.; Donahoo, W.T.; Marosi, K.; Lee, S.A.; Mainous, A.G., 3rd; Leeuwenburgh, C.; Mattson, M.P. Flipping the Metabolic Switch: Understanding and Applying the Health Benefits of Fasting. Obesity 2018, 26, 254–268. [Google Scholar] [CrossRef]
- Manoogian, E.N.C.; Panda, S. Circadian rhythms, time-restricted feeding, and healthy aging. Ageing Res. Rev. 2017, 39, 59–67. [Google Scholar] [CrossRef]
- Mistlberger, R.E.; Lukman, H.; Nadeau, B.G. Circadian rhythms in the Zucker obese rat: Assessment and intervention. Appetite 1998, 30, 255–267. [Google Scholar] [CrossRef]
- Patterson, R.E.; Sears, D.D. Metabolic Effects of Intermittent Fasting. Annu Rev. Nutr. 2017, 37, 371–393. [Google Scholar] [CrossRef] [Green Version]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018, 27, 1212–1221. [Google Scholar] [CrossRef]
- Moro, T.; Tinsley, G.; Bianco, A.; Marcolin, G.; Pacelli, Q.F.; Battaglia, G.; Palma, A.; Gentil, P.; Neri, M.; Paoli, A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl. Med. 2016, 14, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabel, K.; Hoddy, K.K.; Haggerty, N.; Song, J.; Kroeger, C.M.; Trepanowski, J.F.; Panda, S.; Varady, K.A. Effects of 8-h time restricted feeding on body weight and metabolic disease risk factors in obese adults: A pilot study. Nutr. Healthy Aging 2018, 4, 345–353. [Google Scholar] [CrossRef]
- Carlson, O.; Martin, B.; Stote, K.S.; Golden, E.; Maudsley, S.; Najjar, S.S.; Ferrucci, L.; Ingram, D.K.; Longo, D.L.; Rumpler, W.V.; et al. Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal-weight middle-aged men and women. Metab. Clin. Exp. 2007, 56, 1729–1734. [Google Scholar] [CrossRef] [PubMed]
- Stote, K.S.; Baer, D.J.; Spears, K.; Paul, D.R.; Harris, G.K.; Rumpler, W.V.; Strycula, P.; Najjar, S.S.; Ferrucci, L.; Ingram, D.K.; et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am. J. Clin. Nutr. 2007, 85, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, G.M.; Forsse, J.S.; Butler, N.K.; Paoli, A.; Bane, A.A.; La Bounty, P.M.; Morgan, G.B.; Grandjean, P.W. Time-restricted feeding in young men performing resistance training: A randomized controlled trial. Eur. J. Sport Sci. 2017, 17, 200–207. [Google Scholar] [CrossRef]
- Wittenbrink, N.; Ananthasubramaniam, B.; Munch, M.; Koller, B.; Maier, B.; Weschke, C.; Bes, F.; de Zeeuw, J.; Nowozin, C.; Wahnschaffe, A.; et al. High-accuracy determination of internal circadian time from a single blood sample. J. Clin. Investig. 2018, 128, 3826–3839. [Google Scholar] [CrossRef]
- Patterson, R.E.; Laughlin, G.A.; LaCroix, A.Z.; Hartman, S.J.; Natarajan, L.; Senger, C.M.; Martinez, M.E.; Villasenor, A.; Sears, D.D.; Marinac, C.R.; et al. Intermittent Fasting and Human Metabolic Health. J. Acad. Nutr. Diet. 2015, 115, 1203–1212. [Google Scholar] [CrossRef]
- Anson, R.M.; Guo, Z.; de Cabo, R.; Iyun, T.; Rios, M.; Hagepanos, A.; Ingram, D.K.; Lane, M.A.; Mattson, M.P. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc. Natl. Acad. Sci. USA 2003, 100, 6216–6220. [Google Scholar] [CrossRef] [Green Version]
- Mattson, M.P.; Wan, R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J. Nutr. Biochem. 2005, 16, 129–137. [Google Scholar] [CrossRef]
- Froy, O.; Chapnik, N.; Miskin, R. Effect of intermittent fasting on circadian rhythms in mice depends on feeding time. Mech. Ageing Dev. 2009, 130, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Masoro, E.J. Caloric restriction-induced life extension of rats and mice: A critique of proposed mechanisms. Biochim. Biophys. Acta 2009, 1790, 1040–1048. [Google Scholar] [CrossRef]
- Fontana, L.; Nehme, J.; Demaria, M. Caloric restriction and cellular senescence. Mech. Ageing Dev. 2018, 176, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L. Modulating human aging and age-associated diseases. Biochim. Biophys. Acta 2009, 1790, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Wang, H.; Kuang, W. Stem cell rejuvenation and the role of autophagy in age retardation by caloric restriction: An update. Mech. Ageing Dev. 2018, 175, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Katewa, S.D.; Akagi, K.; Bose, N.; Rakshit, K.; Camarella, T.; Zheng, X.; Hall, D.; Davis, S.; Nelson, C.S.; Brem, R.B.; et al. Peripheral Circadian Clocks Mediate Dietary Restriction-Dependent Changes in Lifespan and Fat Metabolism in Drosophila. Cell Metab. 2016, 23, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Challet, E.; Solberg, L.C.; Turek, F.W. Entrainment in calorie-restricted mice: Conflicting zeitgebers and free-running conditions. Am. J. Physiol. 1998, 274, R1751–R1761. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, J.; Graff, C.; Dardente, H.; Pevet, P.; Challet, E. Feeding cues alter clock gene oscillations and photic responses in the suprachiasmatic nuclei of mice exposed to a light/dark cycle. J. Neurosci. 2005, 25, 1514–1522. [Google Scholar] [CrossRef]
- Patel, S.A.; Velingkaar, N.; Makwana, K.; Chaudhari, A.; Kondratov, R. Calorie restriction regulates circadian clock gene expression through BMAL1 dependent and independent mechanisms. Sci. Rep. 2016, 6, 25970. [Google Scholar] [CrossRef]
- Pivovarova, O.; Gogebakan, O.; Sucher, S.; Groth, J.; Murahovschi, V.; Kessler, K.; Osterhoff, M.; Rudovich, N.; Kramer, A.; Pfeiffer, A.F. Regulation of the clock gene expression in human adipose tissue by weight loss. Int. J. Obes. 2016, 40, 899–906. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kessler, K.; Pivovarova-Ramich, O. Meal Timing, Aging, and Metabolic Health. Int. J. Mol. Sci. 2019, 20, 1911. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20081911
Kessler K, Pivovarova-Ramich O. Meal Timing, Aging, and Metabolic Health. International Journal of Molecular Sciences. 2019; 20(8):1911. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20081911
Chicago/Turabian StyleKessler, Katharina, and Olga Pivovarova-Ramich. 2019. "Meal Timing, Aging, and Metabolic Health" International Journal of Molecular Sciences 20, no. 8: 1911. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20081911