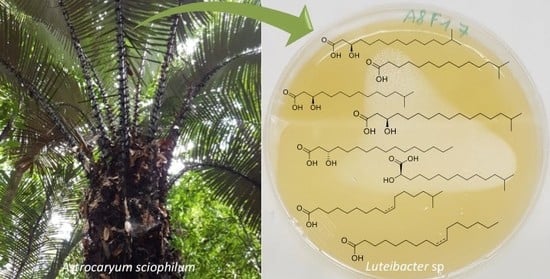
Structural Identification of Antibacterial Lipids from Amazonian Palm Tree Endophytes through the Molecular Network Approach
Abstract
:1. Introduction
2. Results
2.1. Biological Activities of Endophyte Extracts
2.2. Molecular Networking
2.3. Isolated Compounds from Luteibacter sp. BSNB-0721
3. Discussion
4. Materials and Methods
4.1. General
4.2. Endophyte Material
4.3. Identification of Endophytic Strains
4.4. Cultures and Extraction
4.5. UPLC-HRMS Analysis
4.6. MZmine 2.33 Data-Preprocessing Parameters
4.7. Molecular Network Analysis
4.8. Large-Scale Cultivation of Luteibacter sp. and Isolation
4.9. Preparation of Fatty Acid Methyl Esters (FAMEs)
4.10. Determination of Minimal Inhibitory Concentration
4.11. Cytotoxicity Evaluation
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MRSA | Methicillin-resistant Staphylococcus aureus |
| UHPLC-HRMS/MS | Ultra-High Performance Liquid Chromatography-High Resolution Mass Spectrometry |
| MIC | Minimal Inhibitory Concentration |
| EtOAc | Ethyl acetate |
| MN | Molecular Network |
| XIC | Extracted Ion Chromatogram |
| PDA | Potato Dextrose Agar |
| FAME | Fatty Acid Methyl Ester |
| FA | Formic Acid |
| HCl | Hydrochloric acid |
References
- Arnold, A.E.; Mejía, L.C.; Kyllo, D.; Rojas, E.I.; Maynard, Z.; Robbins, N.; Herre, E.A. Fungal endophytes limit pathogen damage in a tropical tree. Proc. Natl. Acad. Sci. USA 2003, 100, 15649–15654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa, L.H.; Vaz, A.B.M.; Caligiorne, R.B.; Campolina, S.; Rosa, C.A. Endophytic fungi associated with the Antarctic grass Deschampsia antarctica Desv. (Poaceae). Polar. Biol. 2009, 32, 161–167. [Google Scholar] [CrossRef]
- Ali, A.H.; Abdelrahman, M.; Radwan, U.; El-Zayat, S.; El-Sayed, M.A. Effect of Thermomyces fungal endophyte isolated from extreme hot desert-adapted plant on heat stress tolerance of cucumber. Appl. Soil Ecol. 2018, 124, 155–162. [Google Scholar] [CrossRef]
- Arnold, A.E.; Lutzoni, F. Diversity and host range of foliar fungal endophytes: Are tropical leaves biodiversity hotspots? Ecology 2007, 88, 541–549. [Google Scholar] [CrossRef]
- Higgins, K.L.; Arnold, A.E.; Coley, P.D.; Kursar, T.A. Communities of fungal endophytes in tropical forest grasses: Highly diverse host- and habitat generalists characterized by strong spatial structure. Fungal Ecol. 2014, 8, 1–11. [Google Scholar] [CrossRef]
- Rosa, L.H.; Vieira, M.L.A.; Cota, B.B.; Johann, S.; Alves, T.M.A.; Zani, C.L.L.; Rosa, C.A. Endophytic Fungi of Tropical Forests: A Promising Source of Bioactive Prototype Molecules for the Treatment of Neglected Diseases. In Drug Development—A Case Study Based Insight into Modern Strategies; Rundfeldt, C., Ed.; In-Tech: Rijeka, Croatia, 2011; pp. 469–486. [Google Scholar]
- Kahn, F. El género Astrocaryum (Arecaceae). Rev. Peru. Biol. 2014, 15, 31–48. [Google Scholar] [CrossRef]
- Charles-dominique, P.; Chave, J.; Dubois, M.A.; De Granville, J.J.; Riera, B.; Vezzoli, C. Colonization front of the understorey palm Astrocaryum sciophilum in a pristine rain forest of French Guiana. Glob. Ecol. Biogeogr. 2003, 12, 237–248. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef]
- MZmine 2: Modular Framework for Processing, Visualizing, and Analyzing Mass Spectrometry-Based Molecular Profile Data. Available online: https://0-bmcbioinformatics-biomedcentral-com.brum.beds.ac.uk/articles/10.1186/1471-2105-11-395 (accessed on 30 August 2018).
- Olivon, F.; Elie, N.; Grelier, G.; Roussi, F.; Litaudon, M.; Touboul, D. MetGem Software for the Generation of Molecular Networks Based on the t-SNE Algorithm. Anal. Chem. 2018, 90, 13900–13908. [Google Scholar] [CrossRef]
- Olivon, F.; Grelier, G.; Roussi, F.; Litaudon, M.; Touboul, D. MZmine 2 Data-Preprocessing To Enhance Molecular Networking Reliability. Anal. Chem. 2017, 89, 7836–7840. [Google Scholar] [CrossRef]
- Vrkoslav, V.; Cvačka, J. Identification of the double-bond position in fatty acid methyl esters by liquid chromatography/atmospheric pressure chemical ionisation mass spectrometry. J. Chromatogr. A 2012, 1259, 244–250. [Google Scholar] [CrossRef]
- Wang, X.; Gong, X.; Li, P.; Lai, D.; Zhou, L. Structural Diversity and Biological Activities of Cyclic Depsipeptides from Fungi. Molecules 2018, 23, 169. [Google Scholar] [CrossRef]
- Li, G.; Kusari, S.; Golz, C.; Strohmann, C.; Spiteller, M. Three cyclic pentapeptides and a cyclic lipopeptide produced by endophytic Fusarium decemcellulare LG53. RSC Adv. 2016, 6, 54092–54098. [Google Scholar] [CrossRef]
- Helaly, S.E.; Kuephadungphan, W.; Phongpaichit, S.; Luangsa-Ard, J.J.; Rukachaisirikul, V.; Stadler, M. Five Unprecedented Secondary Metabolites from the Spider Parasitic Fungus Akanthomyces novoguineensis. Molecules 2017, 22, 991. [Google Scholar] [CrossRef]
- Kuephadungphan, W.; Helaly, S.E.; Daengrot, C.; Phongpaichit, S.; Luangsa-ard, J.J.; Rukachaisirikul, V.; Stadler, M. Akanthopyrones A–D, α-Pyrones Bearing a 4-O-Methyl-β-D-glucopyranose Moiety from the Spider-Associated Ascomycete Akanthomyces novoguineensis. Molecules 2017, 22, 1202. [Google Scholar] [CrossRef]
- Johansen, J.E.; Binnerup, S.J.; Kroer, N.; Mølbak, L. Luteibacter rhizovicinus gen. nov., sp. nov., a yellow-pigmented gammaproteobacterium isolated from the rhizosphere of barley (Hordeum vulgare L.). Int. J. Syst. Evol. Microbiol. 2005, 55, 2285–2291. [Google Scholar] [CrossRef]
- Kämpfer, P.; Lodders, N.; Falsen, E. Luteibacter anthropi sp. nov., isolated from human blood, and reclassification of Dyella yeojuensis Kim et al. 2006 as Luteibacter yeojuensis comb. nov. Int. J. Syst. Evol. Microbiol. 2009, 59, 2884–2887. [Google Scholar] [CrossRef] [PubMed]
- Bergsson, G.; Arnfinnsson, J.; Steingrímsson, O.; Thormar, H. Killing of Gram-positive cocci by fatty acids and monoglycerides. Acta Pathol. Microbiol. Immunol. Scand. 2001, 109, 670–678. [Google Scholar] [CrossRef]
- Parsons, J.B.; Yao, J.; Frank, M.W.; Jackson, P.; Rock, C.O. Membrane Disruption by Antimicrobial Fatty Acids Releases Low-Molecular-Weight Proteins from Staphylococcus aureus. J. Bacteriol. 2012, 194, 5294–5304. [Google Scholar] [CrossRef] [PubMed]
- Kabara, J.J.; Swieczkowski, D.M.; Conley, A.J.; Truant, J.P. Fatty Acids and Derivatives as Antimicrobial Agents. Antimicrob. Agents Chemother. 1972, 2, 23–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kodicek, E.; Worden, A.N. The effect of unsaturated fatty acids on Lactobacillus helveticus and other Gram-positive micro-organisms. Biochem. J. 1945, 39, 78–85. [Google Scholar] [CrossRef]
- Desbois, A.P.; Smith, V. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2009, 85, 1629–1642. [Google Scholar] [CrossRef]
- Casella, T.M.; Eparvier, V.; Mandavid, H.; Bendelac, A.; Odonne, G.; Dayan, L.; Duplais, C.; Espindola, L.S.; Stien, D. Antimicrobial and cytotoxic secondary metabolites from tropical leaf endophytes: Isolation of antibacterial agent pyrrocidine C from Lewia infectoria SNB-GTC2402. Phytochemistry 2013, 96, 370–377. [Google Scholar] [CrossRef]
- Myers, O.D.; Sumner, S.J.; Li, S.; Barnes, S.; Du, X. One Step Forward for Reducing False Positive and False Negative Compound Identifications from Mass Spectrometry Metabolomics Data: New Algorithms for Constructing Extracted Ion Chromatograms and Detecting Chromatographic Peaks. Anal. Chem. 2017, 89, 8696–8703. [Google Scholar] [CrossRef]
- Ichihara, K.; Fukubayashi, Y. Preparation of fatty acid methyl esters for gas-liquid chromatography. J. Lipid Res. 2010, 51, 635–640. [Google Scholar] [CrossRef]
- Rodriguez, A.M.S.; Theodoro, P.; Eparvier, V.; Basset, C.; Silva, M.R.R.; Beauchêne, J.; Espindola, L.; Stien, D. Search for Antifungal Compounds from the Wood of Durable Tropical Trees. J. Nat. Prod. 2010, 73, 1706–1707. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 8th ed.; Approved Standard M7-A8; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009. [Google Scholar]
- Tempête, C.; Werner, G.; Favre, F.; Rojas, A.; Langlois, N. In vitro cytostatic activity of 9-demethoxyporothramycin B. Eur. J. Med. Chem. 1995, 30, 647–650. [Google Scholar] [CrossRef]


| Fatty Acid | MIC on MRSA 1 (µg/mL) | |
|---|---|---|
| 1 |  | 64 |
| 2 |  | 128 |
| 3 |  | 128 |
| 4 |  | 128 |
| 5 |  | ND |
| 6 |  | 128 |
| 7 |  | 32 |
| 8 |  | 64 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barthélemy, M.; Elie, N.; Pellissier, L.; Wolfender, J.-L.; Stien, D.; Touboul, D.; Eparvier, V. Structural Identification of Antibacterial Lipids from Amazonian Palm Tree Endophytes through the Molecular Network Approach. Int. J. Mol. Sci. 2019, 20, 2006. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20082006
Barthélemy M, Elie N, Pellissier L, Wolfender J-L, Stien D, Touboul D, Eparvier V. Structural Identification of Antibacterial Lipids from Amazonian Palm Tree Endophytes through the Molecular Network Approach. International Journal of Molecular Sciences. 2019; 20(8):2006. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20082006
Chicago/Turabian StyleBarthélemy, Morgane, Nicolas Elie, Léonie Pellissier, Jean-Luc Wolfender, Didier Stien, David Touboul, and Véronique Eparvier. 2019. "Structural Identification of Antibacterial Lipids from Amazonian Palm Tree Endophytes through the Molecular Network Approach" International Journal of Molecular Sciences 20, no. 8: 2006. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20082006