Metabolomic Studies of Tissue Injury in Nonhuman Primates Exposed to Gamma-Radiation
Abstract
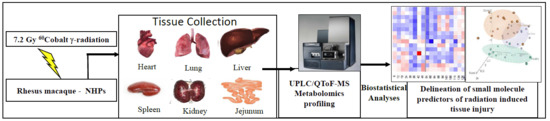
:1. Introduction
2. Results
2.1. Untargeted Metabolomics Analysis Identifies Changes in the Tissue Metabolome of the NHPs Exposed to Radiation
2.2. Metabolite Alterations in the Kidney and Liver Profiles as Indicators of Radiation-Induced Tissue Damage
2.3. Generic Markers of Ionizing Radiation Exposure Across Multiple Tissue Types
3. Discussion
4. Materials and Methods
4.1. Animals and Animal Care
4.2. Radiation Exposure
4.3. Tissue Sample Collection
4.4. Sample Preparation and LC-MS Analyses
4.5. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ohnishi, T. The disaster at Japan’s Fukushima-Daiichi nuclear power plant after the March 11, 2011 earthquake and tsunami, and the resulting spread of radioisotope contamination. Radiat. Res. 2012, 177, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Whitcomb, R.C., Jr.; Ansari, A.J.; Buzzell, J.J.; McCurley, M.C.; Miller, C.W.; Smith, J.M.; Evans, D.L. A public health perspective on the U.S. response to the Fukushima radiological emergency. Health Phys. 2015, 108, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Mettler, F.A., Jr.; Gus’kova, A.K.; Gusev, I. Health effects in those with acute radiation sickness from the Chernobyl accident. Health Phys. 2007, 93, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Hrdina, C.M.; Coleman, C.N.; Bogucki, S.; Bader, J.L.; Hayhurst, R.E.; Forsha, J.D.; Marcozzi, D.; Yeskey, K.; Knebel, A.R. The “RTR” medical response system for nuclear and radiological mass-casualty incidents: A functional TRiage-TReatment-TRansport medical response model. Prehosp. Disaster Med. 2009, 24, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Sproull, M.; Camphausen, K. State-of-the-art advances in radiation biodosimetry for mass casualty events involving radiation exposure. Radiat. Res. 2016, 186, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Amundson, S.A.; Fornace, A.J., Jr. Monitoring human radiation exposure by gene expression profiling: Possibilities and pitfalls. Health Phys. 2003, 85, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.M.; Prasanna, P.G.; Grace, M.B.; Wathen, L.K.; Wallace, R.L.; Koerner, J.F.; Coleman, C.N. Assessment of biodosimetry methods for a mass-casualty radiological incident: Medical response and management considerations. Health Phys. 2013, 105, 540–554. [Google Scholar] [CrossRef]
- Pannkuk, E.L.; Fornace, A.J., Jr.; Laiakis, E.C. Metabolomic applications in radiation biodosimetry: Exploring radiation effects through small molecules. Int. J. Radiat. Biol. 2017, 93, 1151–1176. [Google Scholar] [CrossRef]
- Pannkuk, E.L.; Laiakis, E.C.; Fornace, A.J., Jr.; Fatanmi, O.O.; Singh, V.K. A metabolomic serum signature from nonhuman primates treated with a radiation countermeasure, gamma-tocotrienol, and exposed to ionizing radiation. Health Phys. 2018, 115, 3–11. [Google Scholar] [CrossRef]
- Pannkuk, E.L.; Laiakis, E.C.; Singh, V.K.; Fornace, A.J. Lipidomic signatures of nonhuman primates with radiation-induced hematopoietic syndrome. Sci. Rep. 2017, 7, 9777. [Google Scholar] [CrossRef]
- Singh, V.K.; Newman, V.L.; Romaine, P.L.; Hauer-Jensen, M.; Pollard, H.B. Use of biomarkers for assessing radiation injury and efficacy of countermeasures. Expert Rev. Mol. Diagn. 2016, 16, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Fragkos, K.C.; Forbes, A. Citrulline as a marker of intestinal function and absorption in clinical settings: A systematic review and meta-analysis. United Eur. Gatroenterol. J. 2018, 6, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Pannkuk, E.L.; Laiakis, E.C.; Garcia, M.; Fornace, A.J., Jr.; Singh, V.K. Nonhuman primates with acute radiation syndrome: Results from a global serum metabolomics study after 7.2 Gy total-body irradiation. Radiat. Res. 2018, 190, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Laiakis, E.C.; Mak, T.D.; Strawn, S.J.; Wang, Y.W.; Moon, B.H.; Ake, P.; Fornace, A.J., Jr. Global metabolomic responses in urine from atm deficient mice in response to LD50/30 gamma irradiation doses. Environ. Mol. Mutagen. 2018, 59, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.P.; Kim, Y.M.; Sowa, M.B.; Robinson, R.J.; Gao, X.; Metz, T.O.; Morgan, W.F.; Zhang, Q. Metabolomic response of human skin tissue to low dose ionizing radiation. Mol. Biosyst. 2012, 8, 1979–1986. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Bowen, B.P.; Nguyen, D.H.; Parsa, S.; Huang, Y.; Mao, J.H.; Northen, T.R. Low-dose ionizing radiation-induced blood plasma metabolic response in a diverse genetic mouse population. Radiat. Res. 2012, 178, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Cheema, A.K.; Pathak, R.; Zandkarimi, F.; Kaur, P.; Alkhalil, L.; Singh, R.; Zhong, X.; Ghosh, S.; Aykin-Burns, N.; Hauer-Jensen, M. Liver metabolomics reveals increased oxidative stress and fibrogenic potential in gfrp transgenic mice in response to ionizing radiation. J. Proteome Res. 2014, 13, 3065–3074. [Google Scholar] [CrossRef] [PubMed]
- Batra, V.; Devasagayam, T.P. Interaction between cytotoxic effects of gamma-radiation and folate deficiency in relation to choline reserves. Toxicology 2009, 255, 91–99. [Google Scholar] [CrossRef]
- Goudarzi, M.; Weber, W.M.; Chung, J.; Doyle-Eisele, M.; Melo, D.R.; Mak, T.D.; Strawn, S.J.; Brenner, D.J.; Guilmette, R.; Fornace, A.J., Jr. Serum dyslipidemia is induced by internal exposure to strontium-90 in mice, lipidomic profiling using a data-independent liquid chromatography-mass spectrometry approach. J. Proteome Res. 2015, 14, 4039–4049. [Google Scholar] [CrossRef]
- Nakajima, T.; Ninomiya, Y.; Nenoi, M. Radiation-Induced Reactions in The Liver - Modulation of Radiation Effects by Lifestyle-Related Factors. Int. J. Mol. Sci. 2018, 19, 3855. [Google Scholar] [CrossRef]
- Okunieff, P.; Chen, Y.; Maguire, D.J.; Huser, A.K. Molecular markers of radiation-related normal tissue toxicity. Cancer Metastasis Rev. 2008, 27, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Azzam, E.I.; Jay-Gerin, J.P.; Pain, D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012, 327, 48–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marathe, G.K.; Johnson, C.; Billings, S.D.; Southall, M.D.; Pei, Y.; Spandau, D.; Murphy, R.C.; Zimmerman, G.A.; McIntyre, T.M.; Travers, J.B. Ultraviolet B radiation generates platelet-activating factor-like phospholipids underlying cutaneous damage. J. Biol. Chem. 2005, 280, 35448–35457. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Kelly, J.P.; McGarrah, R.W.; Hellkamp, A.S.; Fiuzat, M.; Testani, J.M.; Wang, T.S.; Verma, A.; Samsky, M.D.; Donahue, M.P.; et al. Prognostic Implications of Long-Chain Acylcarnitines in Heart Failure and Reversibility With Mechanical Circulatory Support. J. Am. Coll. Cardiol. 2016, 67, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Eaton, S.; Bartlett, K.; Pourfarzam, M. Mammalian mitochondrial beta-oxidation. Biochem. J. 1996, 320, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Prithivirajsingh, S.; Story, M.D.; Bergh, S.A.; Geara, F.B.; Ang, K.K.; Ismail, S.M.; Stevens, C.W.; Buchholz, T.A.; Brock, W.A. Accumulation of the common mitochondrial DNA deletion induced by ionizing radiation. FEBS Lett. 2004, 571, 227–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutkowsky, J.M.; Knotts, T.A.; Ono-Moore, K.D.; McCoin, C.S.; Huang, S.; Schneider, D.; Singh, S.; Adams, S.H.; Hwang, D.H. Acylcarnitines activate proinflammatory signaling pathways. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E1378–E1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukhopadhyay, P.; Horvath, B.; Rajesh, M.; Matsumoto, S.; Saito, K.; Batkai, S.; Patel, V.; Tanchian, G.; Gao, R.Y.; Cravatt, B.F.; et al. Fatty acid amide hydrolase is a key regulator of endocannabinoid-induced myocardial tissue injury. Free Radic. Biol. Med. 2011, 50, 179–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, H.Y.; Na, E.J.; Lee, E.; Kwon, Y.; Kim, H.J. Antiepileptic and Neuroprotective Effects of Oleamide in Rat Striatum on Kainate-Induced Behavioral Seizure and Excitotoxic Damage via Calpain Inhibition. Front. Pharmacol. 2017, 8, 817. [Google Scholar] [CrossRef] [PubMed]
- Cravatt, B.F.; Giang, D.K.; Mayfield, S.P.; Boger, D.L.; Lerner, R.A.; Gilula, N.B. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature 1996, 384, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Kulkarni, S.; Fatanmi, O.O.; Wise, S.Y.; Newman, V.L.; Romaine, P.L.; Hendrickson, H.; Gulani, J.; Ghosh, S.P.; Kumar, K.S.; et al. Radioprotective efficacy of gamma-tocotrienol in nonhuman primates. Radiat. Res. 2016, 185, 285–298. [Google Scholar] [CrossRef] [PubMed]
- National Research Council of the National Academy of Sciences. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, DC, USA, 2011.
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 9 October 2018).
- Smith, C.A.; O’Maille, G.; Want, E.J.; Qin, C.; Trauger, S.A.; Brandon, T.R.; Custodio, D.E.; Abagyan, R.; Siuzdak, G. METLIN: A metabolite mass spectral database. Ther. Drug Monit. 2005, 27, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Gil-de-la-Fuente, A.; Godzien, J.; Saugar, S.; Garcia-Carmona, R.; Badran, H.; Wishart, D.S.; Barbas, C.; Otero, A. CEU Mass Mediator 3.0: A Metabolite Annotation Tool. J. Proteome Res. 2019, 18, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vazquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef] [PubMed]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.C.; Muller, M. PROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Therneau, T.M.; Grambsch, P.M. Modeling Survival Data: Extending the Cox Model; Springer-Verlag: New York, NY, USA, 2000. [Google Scholar]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheema, A.K.; Mehta, K.Y.; Rajagopal, M.U.; Wise, S.Y.; Fatanmi, O.O.; Singh, V.K. Metabolomic Studies of Tissue Injury in Nonhuman Primates Exposed to Gamma-Radiation. Int. J. Mol. Sci. 2019, 20, 3360. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20133360
Cheema AK, Mehta KY, Rajagopal MU, Wise SY, Fatanmi OO, Singh VK. Metabolomic Studies of Tissue Injury in Nonhuman Primates Exposed to Gamma-Radiation. International Journal of Molecular Sciences. 2019; 20(13):3360. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20133360
Chicago/Turabian StyleCheema, Amrita K., Khyati Y. Mehta, Meena U. Rajagopal, Stephen Y. Wise, Oluseyi O. Fatanmi, and Vijay K. Singh. 2019. "Metabolomic Studies of Tissue Injury in Nonhuman Primates Exposed to Gamma-Radiation" International Journal of Molecular Sciences 20, no. 13: 3360. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20133360