Surface-Functionalized Nanoparticles as Efficient Tools in Targeted Therapy of Pregnancy Complications
Abstract
:1. Introduction
2. Human Placental Anatomy
3. Nanoparticle Transplacental Transport Mechanisms
3.1. Paracellular Passage
3.2. Transcellular Passage
3.2.1. Endocytosis
3.2.2. Exocytosis
4. Surface-Functionalized Nanoparticles for Targeting the Uterus to Treat Pregnancy Complications
5. Surface-Functionalized Nanoparticles for Targeting the Placenta to Treat Pregnancy Complications
5.1. EGFR Antibody
5.2. Tumor Homing Peptides
5.3. plCSA-BP
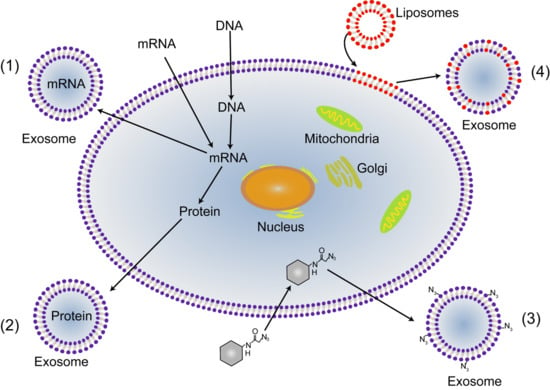
6. Untapped Potential of Placenta-Targeted Tools for Treatment of Pregnancy Complications
7. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CME | Clathrin-mediated endocytosis |
| CVT | Chorionic villous trophoblasts |
| EGFR | Epidermal growth factor receptor |
| eNOS | Endothelial nitric oxide synthase |
| EVT | Extravillous trophoblast |
| FcR | Fc receptors |
| GDM | Gestational diabetes mellitus |
| IgG | Immunoglobulin G |
| IUGR | Intrauterine growth restriction |
| MVB | Multivesicular bodies |
| OTR | Oxytocin receptors |
| PlGF | Placental growth factor |
| PAPP-A | Pregnancy-associated plasma protein-A |
| plCSA | Placental chondroitin sulfate A |
| plCSA-BP | Placental CSA binding peptide |
| sFlt-1 | Soluble FMS-like tyrosine kinase 1 |
References
- Wang, H.; Liddell, C.A.; Coates, M.M.; Mooney, M.D.; Levitz, C.E.; Schumacher, A.E.; Apfel, H.; Iannarone, M.; Phillips, B.; Lofgren, K.T.; et al. Global, regional, and national levels of neonatal, infant, and under-5 mortality during 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 957–979. [Google Scholar] [CrossRef]
- Rodger, M.A.; Betancourt, M.T.; Clark, P.; Lindqvist, P.G.; Dizon-Townson, D.; Said, J.; Seligsohn, U.; Carrier, M.; Salomon, O.; Greer, I.A. The association of factor V leiden and prothrombin gene mutation and placenta-mediated pregnancy complications: A systematic review and meta-analysis of prospective cohort studies. PLoS Med. 2010, 7, e1000292. [Google Scholar] [CrossRef] [PubMed]
- Stillbirth Collaborative Research Network Writing Group. Causes of death among stillbirths. JAMA 2011, 306, 2459–2468. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.S.; Wojdyla, D.; Say, L.; Gülmezoglu, A.M.; Van Look, P.F.A. WHO analysis of causes of maternal death: A systematic review. Lancet 2006, 367, 1066–1074. [Google Scholar] [CrossRef]
- Fisk, N.M.; Atun, R. Market failure and the poverty of new drugs in maternal health. PLoS Med. 2008, 5, e22. [Google Scholar] [CrossRef] [PubMed]
- Fisk, N.M.; McKee, M.; Atun, R. Relative and absolute addressability of global disease burden in maternal and perinatal health by investment in R&D. Trop. Med. Int. Health 2011, 16, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Syme, M.R.; Paxton, J.W.; Keelan, J.A. Drug transfer and metabolism by the human placenta. Clin Pharm. 2004, 43, 487–514. [Google Scholar] [CrossRef] [PubMed]
- Suarez, R.D.; Grobman, W.A.; Parilla, B.V. Indomethacin tocolysis and intraventricular hemorrhage. Obstet. Gynecol. 2001, 97, 921–925. [Google Scholar]
- Kulaga, S.; Sheehy, O.; Zargarzadeh, A.H.; Moussally, K.; Berard, A. Antiepileptic drug use during pregnancy: Perinatal outcomes. Seizure 2011, 20, 667–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venn, A.; Bruinsma, F.; Werther, G.; Pyett, P.; Baird, D.; Jones, P.; Rayner, J.; Lumley, J. Oestrogen treatment to reduce the adult height of tall girls: Long-term effects on fertility. Lancet 2004, 364, 1513–1518. [Google Scholar] [CrossRef]
- Teli, M.K.; Mutalik, S.; Rajanikant, G.K. Nanotechnology and nanomedicine: Going small means aiming big. Curr. Pharm. Des. 2010, 16, 1882–1892. [Google Scholar] [CrossRef] [PubMed]
- Sibley, C.P.; Turner, M.A.; Cetin, I.; Ayuk, P.; Boyd, C.A.; D’Souza, S.W.; Glazier, J.D.; Greenwood, S.L.; Jansson, T.; Powell, T. Placental phenotypes of intrauterine growth. Pediatr. Res. 2005, 58, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y. Doxil(R)—The first FDA-approved nano-drug: Lessons learned. J. Control. Release Off. J. Control. Release Soc. 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Van der Aa, E.M.; Copius Peereboom-Stegeman, J.H.J.; Noordhoek, J.; Gribnau, F.W.J.; Russel, F.G.M. Mechanisms of drug transfer across the human placenta. Pharm. World Sci. 1998, 20, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Gundacker, C.; Neesen, J.; Straka, E.; Ellinger, I.; Dolznig, H.; Hengstschläger, M. Genetics of the human placenta: implications for toxicokinetics. Arch. Toxicol. 2016, 90, 2563–2581. [Google Scholar] [CrossRef] [PubMed]
- Ceckova-Novotna, M.; Pavek, P.; Staud, F. P-glycoprotein in the placenta: Expression, localization, regulation and function. Reprod. Toxicol. 2006, 22, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Enders, A.C.; Blankenship, T.N. Comparative placental structure. Adv. Drug Deliv. Rev. 1999, 38, 3–15. [Google Scholar] [CrossRef]
- Evseenko, D.; Paxton, J.W.; Keelan, J.A. Active transport across the human placenta: Impact on drug efficacy and toxicity. Expert Opin. Drug Metab. Toxicol. 2006, 2, 51–69. [Google Scholar] [CrossRef]
- Ganapathy, V.; Prasad, P.D.; Ganapathy, M.E.; Leibach, F.H. Placental transporters relevant to drug distribution across the maternal-fetal interface. J. Pharmacol. Exp. Ther. 2000, 294, 413–420. [Google Scholar]
- Audus, K.L. Controlling drug delivery across the placenta. Eur. J. Pharm. Sci. 1999, 8, 161–165. [Google Scholar] [CrossRef] [Green Version]
- Keelan, J.A.; Leong, J.W.; Ho, D.; Iyer, K.S. Therapeutic and safety considerations of nanoparticle-mediated drug delivery in pregnancy. Nanomedicine 2015, 10, 2229–2247. [Google Scholar] [CrossRef] [PubMed]
- Menezes, V.; Malek, A.; Keelan, J.A. Nanoparticulate drug delivery in pregnancy: Placental passage and fetal exposure. Curr. Pharm. Biotechnol. 2011, 12, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Saunders, M. Transplacental transport of nanomaterials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Kurz, H.; Fasching, H. The permeation of drugs across the placental barrier. Naunyn-Schmiedebergs Arch. Fur Exp. Pathol. Und Pharmakol. 1968, 259, 214. [Google Scholar] [CrossRef]
- Kertschanska, S.; Schröder, H.; Kaufmann, P. The ultrastructure of the trophoblastic layer of the degu (Octodon degus) Placenta: A Re-evaluation of the ‘Channel Problem’. Placenta 1997, 18, 219–225. [Google Scholar] [CrossRef]
- Kertschanska, S.; Kosanke, G.; Kaufmann, P. Is there morphological evidence for the existence of transtrophoblastic channels in human placental villi? Placenta 1994, 15, 581–596. [Google Scholar] [CrossRef]
- Enders, A.C.; Blankenship, T.N.; Lantz, K.C.; Enders, S.S. Morphological variation in the interhemal areas of chorioallantoic placentae: A review. Placenta 1998, 19, 1–19. [Google Scholar] [CrossRef]
- Bosco, C.; Buffet, C.; Bello, M.A.; Rodrigo, R.; Gutierrez, M.; García, G. Placentation in the degu (Octodon degus): Analogies with extrasubplacental trophoblast and human extravillous trophoblast. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 146, 475–485. [Google Scholar] [CrossRef]
- Kertschanska, S.; Stulcova, B.; Kaufmann, P.; Stulc, J. Distensible transtrophoblastic channels in the rat placenta. Placenta 2000, 21, 670–677. [Google Scholar] [CrossRef]
- Chu, M.; Wu, Q.; Yang, H.; Yuan, R.; Hou, S.; Yang, Y.; Zou, Y.; Xu, S.; Xu, K.; Ji, A.; et al. Transfer of quantum dots from pregnant mice to pups across the placental barrier. Small 2010, 6, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Guschanski, K.; Warnefors, M.; Kaessmann, H. The evolution of duplicate gene expression in mammalian organs. Genome Res. 2017, 27, 1461–1474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulsen, M.S.; Mose, T.; Maroun, L.L.; Mathiesen, L.; Knudsen, L.E.; Rytting, E. Kinetics of silica nanoparticles in the human placenta. Nanotoxicology 2015, 9 (Suppl. 1), 79–86. [Google Scholar] [CrossRef]
- Wick, P.; Malek, A.; Manser, P.; Meili, D.; Maeder-Althaus, X.; Diener, L.; Diener, P.A.; Zisch, A.; Krug, H.F.; von Mandach, U. Barrier capacity of human placenta for nanosized materials. Environ. Health Perspect. 2010, 118, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Menjoge, A.R.; Rinderknecht, A.L.; Navath, R.S.; Faridnia, M.; Kim, C.J.; Romero, R.; Miller, R.K.; Kannan, R.M. Transfer of PAMAM dendrimers across human placenta: Prospects of its use as drug carrier during pregnancy. J. Control. Release Off. J. Control. Release Soc. 2011, 150, 326–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grafmueller, S.; Manser, P.; Diener, L.; Diener, P.A.; Maeder-Althaus, X.; Maurizi, L.; Jochum, W.; Krug, H.F.; Buerki-Thurnherr, T.; von Mandach, U.; et al. Bidirectional Transfer Study of Polystyrene Nanoparticles across the Placental Barrier in an ex Vivo Human Placental Perfusion Model. Env. Health Perspect. 2015, 123, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Kertschanska, S.; Kosanke, G.; Kaufmann, P. Pressure dependence of so-called transtrophoblastic channels during fetal perfusion of human placental villi. Microsc. Res. Tech. 1997, 38, 52–62. [Google Scholar] [CrossRef]
- Leach, L.; Firth, J.A. Structure and permeability of human placental microvasculature. Microsc. Res. Tech. 1997, 38, 137–144. [Google Scholar] [CrossRef]
- Tian, X.; Zhu, M.; Du, L.; Wang, J.; Fan, Z.; Liu, J.; Zhao, Y.; Nie, G. Intrauterine inflammation increases materno-fetal transfer of gold nanoparticles in a size-dependent manner in murine pregnancy. Small 2013, 9, 2432–2439. [Google Scholar] [CrossRef]
- Sakhtianchi, R.; Minchin, R.F.; Lee, K.B.; Alkilany, A.M.; Serpooshan, V.; Mahmoudi, M. Exocytosis of nanoparticles from cells: Role in cellular retention and toxicity. Adv. Colloid Interface Sci. 2013, 201–202, 18–29. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ghosh, R.N.; Maxfield, F.R. Endocytosis. Physiol. Rev. 1997, 77, 759–803. [Google Scholar] [CrossRef] [PubMed]
- Tetro, N.; Moushaev, S.; Rubinchik-Stern, M.; Eyal, S. The Placental Barrier: The Gate and the Fate in Drug Distribution. Pharm. Res. 2018, 35, 71. [Google Scholar] [CrossRef] [PubMed]
- Sahay, G.; Alakhova, D.Y.; Kabanov, A.V. Endocytosis of nanomedicines. J. Control. Release Off. J. Control. Release Soc. 2010, 145, 182–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Stenzel, M.H. Entry of nanoparticles into cells: The importance of nanoparticle properties. Polym. Chem. 2018, 9, 259–272. [Google Scholar] [CrossRef]
- Germain, R.N. An innately interesting decade of research in immunology. Nat. Med. 2004, 10, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ibricevic, A.; Cohen, J.A.; Cohen, J.L.; Gunsten, S.P.; Frechet, J.M.; Walter, M.J.; Welch, M.J.; Brody, S.L. Impact of hydrogel nanoparticle size and functionalization on in vivo behavior for lung imaging and therapeutics. Mol. Pharm. 2009, 6, 1891–1902. [Google Scholar] [CrossRef]
- Choy, M.Y.; Manyonda, I.T. The phagocytic activity of human first trimester extravillous trophoblast. Hum. Reprod. 1998, 13, 2941–2949. [Google Scholar] [CrossRef] [Green Version]
- Bevilacqua, E.; Hoshida, M.S.; Amarante-Paffaro, A.; Albieri-Borges, A.; Zago Gomes, S. Trophoblast phagocytic program: Roles in different placental systems. Int. J. Dev. Biol. 2010, 54, 495–505. [Google Scholar] [CrossRef]
- Matsubara, S.; Takizawa, T.; Yamada, T.; Minakami, H.; Sato, I. Phagocytosis of chorion laeve trophoblasts in patients with chorioamnionitis-associated preterm delivery: Ultrastructural and enzyme-histochemical observations. Placenta 2000, 21, 273–279. [Google Scholar] [CrossRef]
- Conner, S.D.; Schmid, S.L. Regulated portals of entry into the cell. Nature 2003, 422, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Ockleford, C.D.; Whyte, A. Differeniated regions of human placental cell surface associated with exchange of materials between maternal and foetal blood: Coated vesicles. J. Cell Sci. 1977, 25, 293–312. [Google Scholar] [PubMed]
- Kaul, G.; Clemons, T.D.; Iyer, K.S.; Pugazhenthi, K.; Keelan, J.A. Mechanism of uptake of cationic nanoparticles by human placental syncytiotrophoblast cells. Reprod. Sci. 2013, 20, A113. [Google Scholar]
- Rattanapinyopituk, K.; Shimada, A.; Morita, T.; Sakurai, M.; Asano, A.; Hasegawa, T.; Inoue, K.; Takano, H. Demonstration of the clathrin- and caveolin-mediated endocytosis at the maternal-fetal barrier in mouse placenta after intravenous administration of gold nanoparticles. J. Vet. Med. Sci. 2014, 76, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Myllynen, P.K.; Loughran, M.J.; Howard, C.V.; Sormunen, R.; Walsh, A.A.; Vahakangas, K.H. Kinetics of gold nanoparticles in the human placenta. Reprod. Toxicol. 2008, 26, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Jiang, Z.; He, H.; Li, X.; Hu, H.; Zhang, N.; Dai, Y.; Zhou, Z. Uptake and transport of pullulan acetate nanoparticles in the BeWo b30 placental barrier cell model. Int. J. Nanomed. 2018, 13, 4073–4082. [Google Scholar] [CrossRef] [PubMed]
- Ockleford, C.D.; Menon, G. Differentiated regions of human placental cell surface associated with exchange of materials between maternal and foetal blood: A new organelle and the binding of iron. J. Cell Sci. 1977, 25, 279–291. [Google Scholar]
- Dahiya, U.R.; Ganguli, M. Exocytosis—A putative road-block in nanoparticle and nanocomplex mediated gene delivery. J. Control. Release Off. J. Control. Release Soc. 2019. [Google Scholar] [CrossRef]
- Oh, N.; Park, J.H. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int. J. Nanomed. 2014, 9 (Suppl. 1), 51–63. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.; Lei, Y.; Huang, Y.; Zhao, Y.; Li, J.; Huang, T.; Zhang, J.; Wang, J.; Deng, X.; Chen, Z.; et al. Fab fragment glycosylated IgG may play a central role in placental immune evasion. Hum. Reprod. 2015, 30, 380–391. [Google Scholar] [CrossRef]
- Schneider, H.; Miller, R.K. Receptor-mediated uptake and transport of macromolecules in the human placenta. Int. J. Dev. Biol. 2010, 54, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Roopenian, D.C.; Akilesh, S. FcRn: The neonatal Fc receptor comes of age. Nat. Rev. Immunol. 2007, 7, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.L.; West, A.P.; Gan, L.; Bjorkman, P.J. Crystal structure at 2.8 A of an FcRn/heterodimeric Fc complex: Mechanism of pH-dependent binding. Mol. Cell 2001, 7, 867–877. [Google Scholar] [CrossRef]
- Stapleton, N.M.; Armstrong-Fisher, S.S.; Andersen, J.T.; van der Schoot, C.E.; Porter, C.; Page, K.R.; Falconer, D.; de Haas, M.; Williamson, L.M.; Clark, M.R.; et al. Human IgG lacking effector functions demonstrate lower FcRn-binding and reduced transplacental transport. Mol. Immunol. 2018, 95, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, T.; Anderson, C.L.; Robinson, J.M. A novel Fc gamma R-defined, IgG-containing organelle in placental endothelium. J. Immunol. 2005, 175, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, A.-R.; Fuchs, F.; Husslein, P.; Soloff, M.S. Oxytocin receptors in the human uterus during pregnancy and parturition. Am. J. Obstet. Gynecol. 1984, 150, 734–741. [Google Scholar] [CrossRef]
- Wathes, D.C.; Borwick, S.C.; Timmons, P.M.; Leung, S.T.; Thornton, S. Oxytocin receptor expression in human term and preterm gestational tissues prior to and following the onset of labour. J. Endocrinol. 1999, 161, 143–151. [Google Scholar] [CrossRef]
- Adan, R.A.; Van Leeuwen, F.W.; Sonnemans, M.A.; Brouns, M.; Hoffman, G.; Verbalis, J.G.; Burbach, J.P. Rat oxytocin receptor in brain, pituitary, mammary gland, and uterus: Partial sequence and immunocytochemical localization. Endocrinology 1995, 136, 4022–4028. [Google Scholar] [CrossRef]
- Paul, J.W.; Hua, S.; Ilicic, M.; Tolosa, J.M.; Butler, T.; Robertson, S.; Smith, R. Drug delivery to the human and mouse uterus using immunoliposomes targeted to the oxytocin receptor. Am. J. Obs. Gynecol 2017, 216, 283.e1–283.e14. [Google Scholar] [CrossRef]
- Hua, S. Synthesis and in vitro characterization of oxytocin receptor targeted PEGylated immunoliposomes for drug delivery to the uterus. J. Liposome Res. 2019, 1–11. [Google Scholar] [CrossRef]
- Hua, S.; Vaughan, B. In vitro comparison of liposomal drug delivery systems targeting the oxytocin receptor: A potential novel treatment for obstetric complications. Int. J. Nanomed. 2019, 14, 2191–2206. [Google Scholar] [CrossRef] [PubMed]
- Refuerzo, J.S.; Leonard, F.; Bulayeva, N.; Gorenstein, D.; Chiossi, G.; Ontiveros, A.; Longo, M.; Godin, B. Uterus-targeted liposomes for preterm labor management: Studies in pregnant mice. Sci. Rep. 2016, 6, 34710. [Google Scholar] [CrossRef] [PubMed]
- Kaitu’u-Lino, T.J.; Pattison, S.; Ye, L.; Tuohey, L.; Sluka, P.; MacDiarmid, J.; Brahmbhatt, H.; Johns, T.; Horne, A.W.; Brown, J.; et al. Targeted nanoparticle delivery of doxorubicin into placental tissues to treat ectopic pregnancies. Endocrinology 2013, 154, 911–919. [Google Scholar] [CrossRef] [PubMed]
- King, A.; Ndifon, C.; Lui, S.; Widdows, K.; Kotamraju, V.R.; Agemy, L.; Teesalu, T.; Glazier, J.D.; Cellesi, F.; Tirelli, N.; et al. Tumor-homing peptides as tools for targeted delivery of payloads to the placenta. Sci. Adv. 2016, 2, e1600349. [Google Scholar] [CrossRef] [PubMed]
- Cureton, N.; Korotkova, I.; Baker, B.; Greenwood, S.; Wareing, M.; Kotamraju, V.R.; Teesalu, T.; Cellesi, F.; Tirelli, N.; Ruoslahti, E.; et al. Selective Targeting of a Novel Vasodilator to the Uterine Vasculature to Treat Impaired Uteroplacental Perfusion in Pregnancy. Theranostics 2017, 7, 3715–3731. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tan, L.; Yu, Y.; Wang, B.; Chen, Z.; Han, J.; Li, M.; Chen, J.; Xiao, T.; Ambati, B.K.; et al. Placenta-specific drug delivery by trophoblast-targeted nanoparticles in mice. Theranostics 2018, 8, 2765–2781. [Google Scholar] [CrossRef]
- Zhang, B.; Zheng, M.; Cai, L.; Fan, X. Synthesis and Characterization of Placental Chondroitin Sulfate A (plCSA)-Targeting Lipid-Polymer Nanoparticles. J. Vis. Exp. Jove 2018. [Google Scholar] [CrossRef]
- Zhang, B.; Cheng, G.; Zheng, M.; Han, J.; Wang, B.; Li, M.; Chen, J.; Xiao, T.; Zhang, J.; Cai, L.; et al. Targeted delivery of doxorubicin by CSA-binding nanoparticles for choriocarcinoma treatment. Drug Deliv. 2018, 25, 461–471. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Chen, Z.; Han, J.; Li, M.; Nayak, N.R.; Fan, X. Comprehensive Evaluation of the Effectiveness and Safety of Placenta-Targeted Drug Delivery Using Three Complementary Methods. J. Vis. Exp. Jove 2018. [Google Scholar] [CrossRef]
- Chopra, A. Single-Chain Anti-Epidermal Growth Factor Receptor Antibody Fragment Conjugated to Magnetic Iron Oxide Nanoparticles. In Molecular Imaging and Contrast Agent Database (MICAD); National Center for Biotechnology Information: Bethesda, MD, USA, 2004. [Google Scholar]
- Peng, X.H.; Wang, Y.; Huang, D.; Wang, Y.; Shin, H.J.; Chen, Z.; Spewak, M.B.; Mao, H.; Wang, X.; Wang, Y.L.; et al. Targeted delivery of cisplatin to lung cancer using ScFvEGFR-heparin-cisplatin nanoparticles. ACS Nano 2011, 5, 9480–9493. [Google Scholar] [CrossRef]
- Honegger, A. Engineering antibodies for stability and efficient folding. Handb. Exp. Pharmacol. 2008, 47–68. [Google Scholar] [CrossRef]
- Salomon, C.; Rice, G.E. Role of Exosomes in Placental Homeostasis and Pregnancy Disorders. Prog. Mol. Biol. Transl. Sci. 2017, 145, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, S.; Song, J.; Ji, T.; Zhu, M.; Anderson, G.J.; Wei, J.; Nie, G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014, 35, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, B.; Zhang, A.; Hassounah, F.; Seow, Y.; Wood, M.; Ma, F.; Klein, J.D.; Price, S.R.; Wang, X.H. Exosome-Mediated miR-29 Transfer Reduces Muscle Atrophy and Kidney Fibrosis in Mice. Mol. Ther. J. Am. Soc. Gene Ther. 2019, 27, 571–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arrighetti, N.; Corbo, C.; Evangelopoulos, M.; Pasto, A.; Zuco, V.; Tasciotti, E. Exosome-like nanovectors for drug delivery in cancer. Curr. Med. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Kennel, S.J.; Huang, L. Interactions of immunoliposomes with target cells. J. Biol. Chem. 1983, 258, 14034–14040. [Google Scholar] [PubMed]
- Eloy, J.O.; Petrilli, R.; Trevizan, L.N.F.; Chorilli, M. Immunoliposomes: A review on functionalization strategies and targets for drug delivery. Colloids Surf. B Biointerfaces 2017, 159, 454–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Sun, Y.; Liu, Y.; Meng, F.; Lee, R.J. Clinical translation of immunoliposomes for cancer therapy: Recent perspectives. Expert. Opin. Drug Deliv. 2018, 15, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Hansel, T.T.; Kropshofer, H.; Singer, T.; Mitchell, J.A.; George, A.J.T. The safety and side effects of monoclonal antibodies. Nat. Rev. Drug Discov. 2010, 9, 325. [Google Scholar] [CrossRef]
- Challis, J.R.; Lockwood, C.J.; Myatt, L.; Norman, J.E.; Strauss, J.F.; Petraglia, F. Inflammation and Pregnancy. Reprod. Sci. 2009, 16, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Flenady, V.; Reinebrant, H.E.; Liley, H.G.; Tambimuttu, E.G.; Papatsonis, D.N. Oxytocin receptor antagonists for inhibiting preterm labour. Cochrane Database Syst. Rev. 2014, Cd004452. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Jeong, H.Y.; Kim, M.W.; Jeong, I.H.; Choi, M.J.; You, Y.M.; Im, C.S.; Song, I.H.; Lee, T.S.; Park, Y.S. Anti-EGFR lipid micellar nanoparticles co-encapsulating quantum dots and paclitaxel for tumor-targeted theranosis. Nanoscale 2018, 10, 19338–19350. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Huang, W.; Zhang, Z.; Lin, X.; Lin, H.; Peng, L.; Chen, T. Highly Uniform Synthesis of Selenium Nanoparticles with EGFR Targeting and Tumor Microenvironment-Responsive Ability for Simultaneous Diagnosis and Therapy of Nasopharyngeal Carcinoma. ACS Appl. Mater. Interfaces 2019, 11, 11177–11193. [Google Scholar] [CrossRef] [PubMed]
- Groysbeck, N.; Stoessel, A.; Donzeau, M.; da Silva, E.C.; Lehmann, M.; Strub, J.M.; Cianferani, S.; Dembele, K.; Zuber, G. Synthesis and biological evaluation of 2.4 nm thiolate-protected gold nanoparticles conjugated to Cetuximab for targeting glioblastoma cancer cells via the EGFR. Nanotechnology 2019, 30, 184005. [Google Scholar] [CrossRef] [PubMed]
- Beards, F.; Jones, L.E.; Charnock, J.; Forbes, K.; Harris, L.K. Placental Homing Peptide-microRNA Inhibitor Conjugates for Targeted Enhancement of Intrinsic Placental Growth Signaling. Theranostics 2017, 7, 2940–2955. [Google Scholar] [CrossRef] [PubMed]
- Salanti, A.; Dahlback, M.; Turner, L.; Nielsen, M.A.; Barfod, L.; Magistrado, P.; Jensen, A.T.; Lavstsen, T.; Ofori, M.F.; Marsh, K.; et al. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J. Exp. Med. 2004, 200, 1197–1203. [Google Scholar] [CrossRef]
- Salanti, A.; Clausen, T.M.; Agerbaek, M.O.; Al Nakouzi, N.; Dahlback, M.; Oo, H.Z.; Lee, S.; Gustavsson, T.; Rich, J.R.; Hedberg, B.J.; et al. Targeting Human Cancer by a Glycosaminoglycan Binding Malaria Protein. Cancer Cell 2015, 28, 500–514. [Google Scholar] [CrossRef] [Green Version]
- Salanti, A.; Staalsoe, T.; Lavstsen, T.; Jensen, A.T.; Sowa, M.P.; Arnot, D.E.; Hviid, L.; Theander, T.G. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol. Microbiol. 2003, 49, 179–191. [Google Scholar] [CrossRef]
- Resende, M.; Nielsen, M.A.; Dahlback, M.; Ditlev, S.B.; Andersen, P.; Sander, A.F.; Ndam, N.T.; Theander, T.G.; Salanti, A. Identification of glycosaminoglycan binding regions in the Plasmodium falciparum encoded placental sequestration ligand, VAR2CSA. Malar. J. 2008, 7, 104. [Google Scholar] [CrossRef]
- Bastos, N.; Ruivo, C.F.; da Silva, S.; Melo, S.A. Exosomes in cancer: Use them or target them? Semin. Cell Dev. Biol. 2018, 78, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Milane, L.; Singh, A.; Mattheolabakis, G.; Suresh, M.; Amiji, M.M. Exosome mediated communication within the tumor microenvironment. J. Control. Release 2015, 219, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Ohkuchi, A.; Kuwata, T.; Usui, R.; Baba, Y.; Suzuki, H.; Chaw Kyi, T.T.; Matsubara, S.; Saito, S.; Takizawa, T. Endogenous and exogenous miR-520c-3p modulates CD44-mediated extravillous trophoblast invasion. Placenta 2017, 50, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Biro, O.; Fothi, A.; Alasztics, B.; Nagy, B.; Orban, T.I.; Rigo, J., Jr. Circulating exosomal and Argonaute-bound microRNAs in preeclampsia. Gene 2019, 692, 138–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ospina-Prieto, S.; Chaiwangyen, W.; Herrmann, J.; Groten, T.; Schleussner, E.; Markert, U.R.; Morales-Prieto, D.M. MicroRNA-141 is upregulated in preeclamptic placentae and regulates trophoblast invasion and intercellular communication. Transl. Res. J. Lab. Clin. Med. 2016, 172, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Yeo, R.W.Y.; Tan, K.H.; Lim, S.K. Exosomes for drug delivery—A novel application for the mesenchymal stem cell. Biotechnol. Adv. 2013, 31, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, G.; Herrmann, I.K.; Stevens, M.M. Cell-derived vesicles for drug therapy and diagnostics: Opportunities and challenges. Nano Today 2015, 10, 397–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maas, S.L.N.; Breakefield, X.O.; Weaver, A.M. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol. 2017, 27, 172–188. [Google Scholar] [CrossRef]
- Mittelbrunn, M.; Gutiérrez-Vázquez, C.; Villarroya-Beltri, C.; González, S.; Sánchez-Cabo, F.; González, M.Á.; Bernad, A.; Sánchez-Madrid, F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2011, 2, 282. [Google Scholar] [CrossRef] [Green Version]
- Whigham, C.A.; MacDonald, T.M.; Walker, S.P.; Hannan, N.J.; Tong, S.; Kaitu’u-Lino, T.J. The untapped potential of placenta-enriched molecules for diagnostic and therapeutic development. Placenta 2019. [Google Scholar] [CrossRef] [PubMed]
- Turanov, A.A.; Lo, A.; Hassler, M.R.; Makris, A.; Ashar-Patel, A.; Alterman, J.F.; Coles, A.H.; Haraszti, R.A.; Roux, L.; Godinho, B.M.D.C.; et al. RNAi modulation of placental sFLT1 for the treatment of preeclampsia. Nat. Biotechnol. 2018, 36, 1164. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; de Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef] [PubMed]
- Muoth, C.; Aengenheister, L.; Kucki, M.; Wick, P.; Buerki-Thurnherr, T. Nanoparticle transport across the placental barrier: Pushing the field forward! Nanomedicine 2016, 11, 941–957. [Google Scholar] [CrossRef]



| Decorated Ligands/Peptides | Nanoparticles Type | Conjugated Method | Targeted Organ | Pregnancy Complications | Ref. |
|---|---|---|---|---|---|
| OTR-antibody | Immunoliposomes | Michael-type addition reaction | Uterus | Preterm birth | [69,70,71] |
| Atosiban | Liposomes | Post-insertion technique | Uterus | Preterm birth | [71,72] |
| EGFR antibody | Nanocells | Bispecific antibodies | Placenta | Ectopic pregnancies | [73] |
| Tumor homing peptides | Liposomes | Michael-type addition reaction | Placenta | Fetal growth restriction | [74] |
| Uteroplacental-targeted peptide | Liposomes | Michael-type addition reaction | Placenta | Fetal growth restriction | [75] |
| plCSA-BP | Lipid-polymer nanoparticles | EDC/NHS | Placenta | Normal pregnancy, Choriocarcinoma | [76,77,78,79] |
| ScFvEGFR antibody | Untapped | EDC/NHS | Placenta | Untapped | [80,81,82] |
| Untapped | Placenta-derived exosomes | Untapped | Placenta | Untapped | [83,84,85,86,87] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Liang, R.; Zheng, M.; Cai, L.; Fan, X. Surface-Functionalized Nanoparticles as Efficient Tools in Targeted Therapy of Pregnancy Complications. Int. J. Mol. Sci. 2019, 20, 3642. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20153642
Zhang B, Liang R, Zheng M, Cai L, Fan X. Surface-Functionalized Nanoparticles as Efficient Tools in Targeted Therapy of Pregnancy Complications. International Journal of Molecular Sciences. 2019; 20(15):3642. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20153642
Chicago/Turabian StyleZhang, Baozhen, Ruijing Liang, Mingbin Zheng, Lintao Cai, and Xiujun Fan. 2019. "Surface-Functionalized Nanoparticles as Efficient Tools in Targeted Therapy of Pregnancy Complications" International Journal of Molecular Sciences 20, no. 15: 3642. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20153642