The Mode-of-Action of Targeted Alpha Therapy Radium-223 as an Enabler for Novel Combinations to Treat Patients with Bone Metastasis
Abstract
:1. Introduction
2. Disseminated Tumor Cells and Dormancy in Bone Metastasis
3. The Tumor Microenvironment in Bone Metastasis
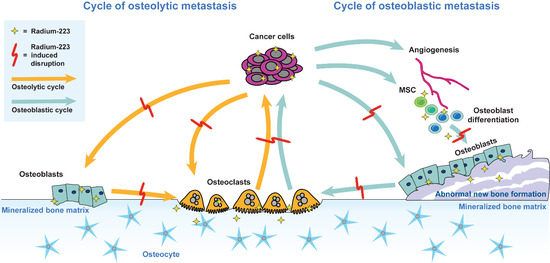
3.1. The Vicious Cycle of Osteolytic Bone Metastasis
3.2. The Vicious Cycle of Osteoblastic Bone Metastasis
4. Bone Supportive Agents
4.1. Bisphosphonates
4.2. RANKL Inhibitor
5. Mechanism of Action and Combination Potential of Radium-223
5.1. Radium-223 Therapy in Combination with DNA Damage Repair Inhibitors
5.2. Radium-223 Therapy in Combination with Immuno-Oncological Treatments
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, M.R. Skeletal-related events in prostate cancer. Clin. Adv. Hematol. Oncol. H O 2012, 10, 535–536. [Google Scholar] [PubMed]
- Coleman, R.E. The role of bone markers in metastatic bone disease. Cancer Treat. Rev. 2006, 32 (Suppl. 1), 1–2. [Google Scholar] [CrossRef]
- Cetin, K.; Christiansen, C.F.; Svaerke, C.; Jacobsen, J.B.; Sorensen, H.T. Survival in patients with breast cancer with bone metastasis: A Danish population-based cohort study on the prognostic impact of initial stage of disease at breast cancer diagnosis and length of the bone metastasis-free interval. BMJ Open 2015, 5, e007702. [Google Scholar] [CrossRef] [PubMed]
- Som, A.; Tu, S.M.; Liu, J.; Wang, X.; Qiao, W.; Logothetis, C.; Corn, P.G. Response in bone turnover markers during therapy predicts overall survival in patients with metastatic prostate cancer: Analysis of three clinical trials. Br. J. Cancer 2012, 107, 1547–1553. [Google Scholar] [CrossRef] [PubMed]
- Tait, C.; Moore, D.; Hodgson, C.; Brown, M.; Morris, T.; Growcott, J.; Malone, M.; Hughes, A.; Renehan, A.; Clarke, N.W.; et al. Quantification of skeletal metastases in castrate-resistant prostate cancer predicts progression-free and overall survival. BJU Int. 2014, 114, E70–E73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fizazi, K.; Massard, C.; Smith, M.; Rader, M.; Brown, J.; Milecki, P.; Shore, N.; Oudard, S.; Karsh, L.; Carducci, M.; et al. Bone-related Parameters are the Main Prognostic Factors for Overall Survival in Men with Bone Metastases from Castration-resistant Prostate Cancer. Eur. Urol. 2015, 68, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Gobbini, E.; Ezzalfani, M.; Dieras, V.; Bachelot, T.; Brain, E.; Debled, M.; Jacot, W.; Mouret-Reynier, M.A.; Goncalves, A.; Dalenc, F.; et al. Time trends of overall survival among metastatic breast cancer patients in the real-life ESME cohort. Eur. J. Cancer 2018, 96, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Pezaro, C.; Omlin, A.; Lorente, D.; Rodrigues, D.N.; Ferraldeschi, R.; Bianchini, D.; Mukherji, D.; Riisnaes, R.; Altavilla, A.; Crespo, M.; et al. Visceral disease in castration-resistant prostate cancer. Eur. Urol. 2014, 65, 270–273. [Google Scholar] [CrossRef]
- Tannock, I.F.; de Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Theodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 2004, 351, 1502–1512. [Google Scholar] [CrossRef]
- Freedland, S.J.; Richhariya, A.; Wang, H.; Chung, K.; Shore, N.D. Treatment patterns in patients with prostate cancer and bone metastasis among US community-based urology group practices. Urology 2012, 80, 293–298. [Google Scholar] [CrossRef]
- Savci-Heijink, C.D.; Halfwerk, H.; Hooijer, G.K.; Horlings, H.M.; Wesseling, J.; van de Vijver, M.J. Retrospective analysis of metastatic behaviour of breast cancer subtypes. Breast Cancer Res. Treat. 2015, 150, 547–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coleman, R.E. Skeletal complications of malignancy. Cancer 1997, 80, 1588–1594. [Google Scholar] [CrossRef]
- Henriksen, G.; Breistol, K.; Bruland, O.S.; Fodstad, O.; Larsen, R.H. Significant antitumor effect from bone-seeking, alpha-particle-emitting (223)Ra demonstrated in an experimental skeletal metastases model. Cancer Res. 2002, 62, 3120–3125. [Google Scholar] [PubMed]
- Salmon, P.L.; Onischuk, Y.N.; Bondarenko, O.A.; Lanyon, L.E. Alpha-particle doses to cells of the bone remodeling cycle from alpha-particle-emitting bone-seekers: Indications of an antiresorptive effect of actinides. Radiat. Res. 1999, 152, S43–S47. [Google Scholar] [CrossRef] [PubMed]
- Suominen, M.I.; Rissanen, J.P.; Kakonen, R.; Fagerlund, K.M.; Alhoniemi, E.; Mumberg, D.; Ziegelbauer, K.; Halleen, J.M.; Kakonen, S.M.; Scholz, A. Survival benefit with radium-223 dichloride in a mouse model of breast cancer bone metastasis. J. Natl. Cancer Inst. 2013, 105, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Suominen, M.I.; Fagerlund, K.M.; Rissanen, J.P.; Konkol, Y.M.; Morko, J.P.; Peng, Z.; Alhoniemi, E.J.; Laine, S.K.; Corey, E.; Mumberg, D.; et al. Radium-223 Inhibits Osseous Prostate Cancer Growth by Dual Targeting of Cancer Cells and Bone Microenvironment in Mouse Models. Clin. Cancer Res. 2017, 23, 4335–4346. [Google Scholar] [CrossRef] [PubMed]
- Darvin, P.; Toor, S.M.; Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors: Recent progress and potential biomarkers. Exp. Mol. Med. 2018, 50, 165. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Parker, C.C.; Tombal, B.F.; Miller, K.; Saad, F.; Shen, J.; Zhang, A.; Kornacker, M.; Higano, C.S. ERA 223: A phase 3 trial of radium-223 dichloride in combination with abiraterone acetate and prednisone in the treatment of asymptomatic or mildly symptomatic chemotherapy-naïve patients with bone-predominant metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2016, 34, TPS5088. [Google Scholar] [CrossRef]
- Tombal, B.F.; Loriot, Y.; Saad, F.; McDermott, R.S.; Elliott, T.; Rodriguez-Vida, A.; Nole, F.; Fournier, B.; Collette, L.; Gillessen, S. Decreased fracture rate by mandating bone-protecting agents in the EORTC 1333/PEACE III trial comparing enzalutamide and Ra223 versus enzalutamide alone: An interim safety analysis. In Proceedings of the 2019 ASCO Annual Meeting, Chicago, IL, USA, 31 May–4 June 2019. [Google Scholar]
- Bao, B.; Ahmad, A.; Azmi, A.S.; Ali, S.; Sarkar, F.H. Overview of cancer stem cells (CSCs) and mechanisms of their regulation: Implications for cancer therapy. Curr. Protoc. Pharmacol. 2013, 61, 14–25. [Google Scholar] [CrossRef]
- Morgan, T.M.; Lange, P.H.; Porter, M.P.; Lin, D.W.; Ellis, W.J.; Gallaher, I.S.; Vessella, R.L. Disseminated tumor cells in prostate cancer patients after radical prostatectomy and without evidence of disease predicts biochemical recurrence. Clin. Cancer Res. 2009, 15, 677–683. [Google Scholar] [CrossRef]
- Braun, S.; Vogl, F.D.; Naume, B.; Janni, W.; Osborne, M.P.; Coombes, R.C.; Schlimok, G.; Diel, I.J.; Gerber, B.; Gebauer, G.; et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N. Engl. J. Med. 2005, 353, 793–802. [Google Scholar] [CrossRef]
- Hartkopf, A.D.; Stefanescu, D.; Wallwiener, M.; Hahn, M.; Becker, S.; Solomayer, E.F.; Fehm, T.N.; Brucker, S.Y.; Taran, F.A. Tumor cell dissemination to the bone marrow and blood is associated with poor outcome in patients with metastatic breast cancer. Breast Cancer Res. Treat. 2014, 147, 345–351. [Google Scholar] [CrossRef]
- Sanger, N.; Effenberger, K.E.; Riethdorf, S.; Van Haasteren, V.; Gauwerky, J.; Wiegratz, I.; Strebhardt, K.; Kaufmann, M.; Pantel, K. Disseminated tumor cells in the bone marrow of patients with ductal carcinoma in situ. Int. J. Cancer 2011, 129, 2522–2526. [Google Scholar] [CrossRef]
- Ottewell, P.D.; Wang, N.; Brown, H.K.; Reeves, K.J.; Fowles, C.A.; Croucher, P.I.; Eaton, C.L.; Holen, I. Zoledronic acid has differential antitumor activity in the pre- and postmenopausal bone microenvironment in vivo. Clin. Cancer Res. 2014, 20, 2922–2932. [Google Scholar] [CrossRef]
- Ottewell, P.D.; Wang, N.; Meek, J.; Fowles, C.A.; Croucher, P.I.; Eaton, C.L.; Holen, I. Castration-induced bone loss triggers growth of disseminated prostate cancer cells in bone. Endocr. Relat. Cancer 2014, 21, 769–781. [Google Scholar] [CrossRef]
- Hensel, J.; Thalmann, G.N. Biology of Bone Metastases in Prostate Cancer. Urology 2016, 92, 6–13. [Google Scholar] [CrossRef] [Green Version]
- Ghajar, C.M.; Peinado, H.; Mori, H.; Matei, I.R.; Evason, K.J.; Brazier, H.; Almeida, D.; Koller, A.; Hajjar, K.A.; Stainier, D.Y.; et al. The perivascular niche regulates breast tumour dormancy. Nat. Cell Biol. 2013, 15, 807–817. [Google Scholar] [CrossRef]
- Ghajar, C.M. Disseminated Tumor Cell Dormancy by distant microenvironments (abstract). In Proceedings of the 107th Annual Meeting of American Association of Cancer Research, New Orleans, LA, USA, 16–20 April 2016. [Google Scholar]
- Calvi, L.M. Osteoblastic activation in the hematopoietic stem cell niche. Ann. N. Y. Acad. Sci. 2006, 1068, 477–488. [Google Scholar] [CrossRef]
- Shiozawa, Y.; Pienta, K.J.; Taichman, R.S. Hematopoietic stem cell niche is a potential therapeutic target for bone metastatic tumors. Clin. Cancer Res. 2011, 17, 5553–5558. [Google Scholar] [CrossRef]
- Ren, G.; Esposito, M.; Kang, Y. Bone metastasis and the metastatic niche. J. Mol. Med. 2015, 93, 1203–1212. [Google Scholar] [CrossRef] [Green Version]
- Giles, A.J.; Reid, C.M.; Evans, J.D.; Murgai, M.; Vicioso, Y.; Highfill, S.L.; Kasai, M.; Vahdat, L.; Mackall, C.L.; Lyden, D.; et al. Activation of Hematopoietic Stem/Progenitor Cells Promotes Immunosuppression Within the Pre-metastatic Niche. Cancer Res. 2016, 76, 1335–1347. [Google Scholar] [CrossRef]
- Giannoni, E.; Bianchini, F.; Calorini, L.; Chiarugi, P. Cancer associated fibroblasts exploit reactive oxygen species through a proinflammatory signature leading to epithelial mesenchymal transition and stemness. Antioxid. Redox Signal. 2011, 14, 2361–2371. [Google Scholar] [CrossRef]
- Barcellos-de-Souza, P.; Comito, G.; Pons-Segura, C.; Taddei, M.L.; Gori, V.; Becherucci, V.; Bambi, F.; Margheri, F.; Laurenzana, A.; Del Rosso, M.; et al. Mesenchymal Stem Cells are Recruited and Activated into Carcinoma-Associated Fibroblasts by Prostate Cancer Microenvironment-Derived TGF-beta1. Stem Cells 2016, 34, 2536–2547. [Google Scholar] [CrossRef]
- Mundy, G.R. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2002, 2, 584–593. [Google Scholar] [CrossRef]
- Guise, T.A.; Mohammad, K.S.; Clines, G.; Stebbins, E.G.; Wong, D.H.; Higgins, L.S.; Vessella, R.; Corey, E.; Padalecki, S.; Suva, L.; et al. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin. Cancer Res. 2006, 12, 6213s–6216s. [Google Scholar] [CrossRef]
- Guise, T.A.; Yin, J.J.; Taylor, S.D.; Kumagai, Y.; Dallas, M.; Boyce, B.F.; Yoneda, T.; Mundy, G.R. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J. Clin. Investig. 1996, 98, 1544–1549. [Google Scholar] [CrossRef]
- Morgan, H.; Tumber, A.; Hill, P.A. Breast cancer cells induce osteoclast formation by stimulating host IL-11 production and downregulating granulocyte/macrophage colony-stimulating factor. Int. J. Cancer 2004, 109, 653–660. [Google Scholar] [CrossRef]
- Sotiriou, C.; Lacroix, M.; Lespagnard, L.; Larsimont, D.; Paesmans, M.; Body, J.J. Interleukins-6 and -11 expression in primary breast cancer and subsequent development of bone metastases. Cancer Lett. 2001, 169, 87–95. [Google Scholar] [CrossRef]
- Cox, T.R.; Rumney, R.M.H.; Schoof, E.M.; Perryman, L.; Hoye, A.M.; Agrawal, A.; Bird, D.; Latif, N.A.; Forrest, H.; Evans, H.R.; et al. The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature 2015, 522, 106–110. [Google Scholar] [CrossRef] [Green Version]
- Juarez, P.; Guise, T.A. TGF-beta in cancer and bone: Implications for treatment of bone metastases. Bone 2011, 48, 23–29. [Google Scholar] [CrossRef]
- Saidak, Z.; Boudot, C.; Abdoune, R.; Petit, L.; Brazier, M.; Mentaverri, R.; Kamel, S. Extracellular calcium promotes the migration of breast cancer cells through the activation of the calcium sensing receptor. Exp. Cell Res. 2009, 315, 2072–2080. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Takyar, F.M.; Swan, K.; Jeong, J.; VanHouten, J.; Sullivan, C.; Dann, P.; Yu, H.; Fiaschi-Taesch, N.; Chang, W.; et al. Calcium-Sensing Receptor Promotes Breast Cancer by Stimulating Intracrine Actions of Parathyroid Hormone-Related Protein. Cancer Res. 2016, 76, 5348–5360. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.J.; Kajiya, M.; AlShwaimi, E.; Sasaki, H.; Hong, J.; Ok, P.; Rezende, T.M.; Pagonis, T.C.; White, R.R.; Paster, B.J.; et al. Bacteria-reactive immune response may induce RANKL-expressing T cells in the mouse periapical bone loss lesion. J. Endod. 2012, 38, 346–350. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, L.; Roato, I. The Impact of Immune System in Regulating Bone Metastasis Formation by Osteotropic Tumors. J. Immunol. Res. 2015, 2015, 143526. [Google Scholar] [CrossRef] [PubMed]
- Rehault, S.; Monget, P.; Mazerbourg, S.; Tremblay, R.; Gutman, N.; Gauthier, F.; Moreau, T. Insulin-like growth factor binding proteins (IGFBPs) as potential physiological substrates for human kallikreins hK2 and hK3. Eur. J. Biochem. 2001, 268, 2960–2968. [Google Scholar] [CrossRef]
- Cramer, S.D.; Chen, Z.; Peehl, D.M. Prostate specific antigen cleaves parathyroid hormone-related protein in the PTH-like domain: Inactivation of PTHrP-stimulated cAMP accumulation in mouse osteoblasts. J. Urol. 1996, 156, 526–531. [Google Scholar] [CrossRef]
- Cumming, A.P.; Hopmans, S.N.; Vukmirovic-Popovic, S.; Duivenvoorden, W.C. PSA affects prostate cancer cell invasion in vitro and induces an osteoblastic phenotype in bone in vivo. Prostate Cancer Prostatic Dis. 2011, 14, 286–294. [Google Scholar] [CrossRef] [Green Version]
- Mountzios, G.; Terpos, E.; Syrigos, K.; Papadimitriou, C.; Papadopoulos, G.; Bamias, A.; Mavrikakis, M.; Dimopoulos, M.A. Markers of bone remodeling and skeletal morbidity in patients with solid tumors metastatic to the skeleton receiving the biphosphonate zoledronic acid. Transl. Res. 2010, 155, 247–255. [Google Scholar] [CrossRef]
- Roato, I.; D’Amelio, P.; Gorassini, E.; Grimaldi, A.; Bonello, L.; Fiori, C.; Delsedime, L.; Tizzani, A.; De Libero, A.; Isaia, G.; et al. Osteoclasts are active in bone forming metastases of prostate cancer patients. PLoS ONE 2008, 3, e3627. [Google Scholar] [CrossRef]
- Yin, J.J.; Mohammad, K.S.; Kakonen, S.M.; Harris, S.; Wu-Wong, J.R.; Wessale, J.L.; Padley, R.J.; Garrett, I.R.; Chirgwin, J.M.; Guise, T.A. A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proc. Natl. Acad. Sci. USA 2003, 100, 10954–10959. [Google Scholar] [CrossRef] [Green Version]
- Coxon, F.P.; Thompson, K.; Roelofs, A.J.; Ebetino, F.H.; Rogers, M.J. Visualizing mineral binding and uptake of bisphosphonate by osteoclasts and non-resorbing cells. Bone 2008, 42, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; McCloskey, E.V.; Johansson, H.; Cooper, C.; Rizzoli, R.; Reginster, J.Y. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2013, 24, 23–57. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Parker, C.; Saad, F.; Miller, K.; Tombal, B.; Ng, Q.S.; Boegemann, M.; Matveev, V.; Piulats, J.M.; Zucca, L.E.; et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 408–419. [Google Scholar] [CrossRef]
- Coleman, R.; Cameron, D.; Dodwell, D.; Bell, R.; Wilson, C.; Rathbone, E.; Keane, M.; Gil, M.; Burkinshaw, R.; Grieve, R.; et al. Adjuvant zoledronic acid in patients with early breast cancer: Final efficacy analysis of the AZURE (BIG 01/04) randomised open-label phase 3 trial. Lancet Oncol. 2014, 15, 997–1006. [Google Scholar] [CrossRef]
- Gnant, M.; Mlineritsch, B.; Stoeger, H.; Luschin-Ebengreuth, G.; Heck, D.; Menzel, C.; Jakesz, R.; Seifert, M.; Hubalek, M.; Pristauz, G.; et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol. 2011, 12, 631–641. [Google Scholar] [CrossRef]
- Wilson, C.; Hinsley, S.; Marshall, H.; Cameron, D.; Bell, R.; Dodwell, D.; Coleman, R.E. Reproductive hormone analyses and effects of adjuvant zoledronic acid in early breast cancer—An AZURE (BIG 01/04) sub-study. J. Bone Oncol. 2017, 9, 48–54. [Google Scholar] [CrossRef]
- Lipton, A.; Chapman, J.W.; Leitzel, K.; Garg, A.; Pritchard, K.I.; Ingle, J.N.; Budd, G.T.; Ellis, M.J.; Sledge, G.W.; Rabaglio, M.; et al. Osteoporosis therapy and outcomes for postmenopausal patients with hormone receptor-positive breast cancer: NCIC CTG MA.27. Cancer 2017, 123, 2444–2451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fizazi, K.; Carducci, M.; Smith, M.; Damiao, R.; Brown, J.; Karsh, L.; Milecki, P.; Shore, N.; Rader, M.; Wang, H.; et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet 2011, 377, 813–822. [Google Scholar] [CrossRef]
- Lipton, A.; Fizazi, K.; Stopeck, A.T.; Henry, D.H.; Brown, J.E.; Yardley, D.A.; Richardson, G.E.; Siena, S.; Maroto, P.; Clemens, M.; et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: A combined analysis of 3 pivotal, randomised, phase 3 trials. Eur. J. Cancer 2012, 48, 3082–3092. [Google Scholar] [CrossRef]
- Smith, M.R.; Saad, F.; Oudard, S.; Shore, N.; Fizazi, K.; Sieber, P.; Tombal, B.; Damiao, R.; Marx, G.; Miller, K.; et al. Denosumab and bone metastasis-free survival in men with nonmetastatic castration-resistant prostate cancer: Exploratory analyses by baseline prostate-specific antigen doubling time. J. Clin. Oncol. 2013, 31, 3800–3806. [Google Scholar] [CrossRef]
- Gnant, M.; Pfeiler, G.; Dubsky, P.C.; Hubalek, M.; Greil, R.; Jakesz, R.; Wette, V.; Balic, M.; Haslbauer, F.; Melbinger, E.; et al. Adjuvant denosumab in breast cancer (ABCSG-18): A multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2015, 386, 433–443. [Google Scholar] [CrossRef]
- Gnant, M.; Pfeiler, G.; Steger, G.G.; Egle, D.; Greil, R.; Fitzal, F.; Wette, V.; Balic, M.; Haslbauer, F.; Melbinger-Zeinitzer, E.; et al. Adjuvant denosumab in postmenopausal patients with hormone receptor-positive breast cancer (ABCSG-18): Disease-free survival results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 339–351. [Google Scholar] [CrossRef]
- Brechbiel, M.W. Targeted alpha-therapy: Past, present, future? Dalton Trans. 2007, 4918–4928. [Google Scholar] [CrossRef]
- Targeted Alpha Therapy Working, G.; Parker, C.; Lewington, V.; Shore, N.; Kratochwil, C.; Levy, M.; Linden, O.; Noordzij, W.; Park, J.; Saad, F. Targeted Alpha Therapy, an Emerging Class of Cancer Agents: A Review. JAMA Oncol. 2018, 4, 1765–1772. [Google Scholar] [CrossRef]
- Sgouros, G.; Roeske, J.C.; McDevitt, M.R.; Palm, S.; Allen, B.J.; Fisher, D.R.; Brill, A.B.; Song, H.; Howell, R.W.; Akabani, G.; et al. MIRD Pamphlet No. 22 (abridged): Radiobiology and dosimetry of alpha-particle emitters for targeted radionuclide therapy. J. Nucl. Med. 2010, 51, 311–328. [Google Scholar] [CrossRef]
- Nevedomskaya, E.; Baumgart, S.J.; Haendler, B. Recent Advances in Prostate Cancer Treatment and Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1359. [Google Scholar] [CrossRef]
- Du, Y.; Carrio, I.; De Vincentis, G.; Fanti, S.; Ilhan, H.; Mommsen, C.; Nitzsche, E.; Sundram, F.; Vogel, W.; Oyen, W.; et al. Practical recommendations for radium-223 treatment of metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1671–1678. [Google Scholar] [CrossRef]
- Marques, I.A.; Neves, A.R.; Abrantes, A.M.; Pires, A.S.; Tavares-da-Silva, E.; Figueiredo, A.; Botelho, M.F. Targeted alpha therapy using Radium-223: From physics to biological effects. Cancer Treat. Rev. 2018, 68, 47–54. [Google Scholar] [CrossRef]
- Dekempeneer, Y.; Keyaerts, M.; Krasniqi, A.; Puttemans, J.; Muyldermans, S.; Lahoutte, T.; D’Huyvetter, M.; Devoogdt, N. Targeted alpha therapy using short-lived alpha-particles and the promise of nanobodies as targeting vehicle. Expert Opin. Biol. Ther. 2016, 16, 1035–1047. [Google Scholar] [CrossRef] [Green Version]
- Kozempel, J.; Mokhodoeva, O.; Vlk, M. Progress in Targeted Alpha-Particle Therapy. What We Learned about Recoils Release from In Vivo Generators. Molecules 2018, 23, 581. [Google Scholar] [CrossRef]
- Jadvar, H.; Quinn, D.I. Targeted alpha-particle therapy of bone metastases in prostate cancer. Clin. Nucl. Med. 2013, 38, 966–971. [Google Scholar] [CrossRef]
- Pouget, J.P.; Navarro-Teulon, I.; Bardies, M.; Chouin, N.; Cartron, G.; Pelegrin, A.; Azria, D. Clinical radioimmunotherapy--the role of radiobiology. Nat. Rev. Clin. Oncol. 2011, 8, 720–734. [Google Scholar] [CrossRef]
- Baczyk, M. Radioisotope therapy of bone metastases. Nucl. Med. Rev. 2011, 14, 96–104. [Google Scholar] [CrossRef] [Green Version]
- Kassis, A.I.; Adelstein, S.J. Radiobiologic principles in radionuclide therapy. J. Nucl. Med. 2005, 46 (Suppl. 1), 4S–12S. [Google Scholar]
- Larsen, R.H.; Saxtorph, H.; Skydsgaard, M.; Borrebaek, J.; Jonasdottir, T.J.; Bruland, O.S.; Klastrup, S.; Harling, R.; Ramdahl, T. Radiotoxicity of the alpha-emitting bone-seeker 223Ra injected intravenously into mice: Histology, clinical chemistry and hematology. In Vivo 2006, 20, 325–331. [Google Scholar]
- Malamas, A.S.; Gameiro, S.R.; Knudson, K.M.; Hodge, J.W. Sublethal exposure to alpha radiation (223Ra dichloride) enhances various carcinomas’ sensitivity to lysis by antigen-specific cytotoxic T lymphocytes through calreticulin-mediated immunogenic modulation. Oncotarget 2016, 7, 86937–86947. [Google Scholar] [CrossRef]
- Suominen, M.I.; Kähkönen, T.; Mäki-Jouppila, J.; Rissanen, J.P.; Luostarinen, A.; Fagerlund, K.M.; Huhtinen, A.; Sjöholm, B.; Alhoniemi, E.; Siemeiste, G.; et al. Ra-223 and bortezomib combination in a MM model. In Proceedings of the American Association for Cancer Research Annual Meeting 2017, Washington, DC, USA, 1–5 April 2017; AACR: Philadelphia, PA, USA, 2017. [Google Scholar]
- Wengner, A.M.; Siemeister, G.; Luecking, U.; Lefranc, J.; Scholz, A.; Suominen, M.; Meyer, K.; Lagkadinou, E.; Mumberg, D. Synergistic in vivo activity of the ATR inhibitor BAY 1895344 in combination with the targeted alpha therapy radium-223 dichloride in a preclinical tumor model mimicking bone metastatic castration-resistant prostate cancer (mCRPC). In Proceedings of the American Association for Cancer Research Annual Meeting 2018, Chicago, IL, USA, 14–18 April 2018; AACR: Philadelphia, PA, USA, 2018. [Google Scholar]
- Wickstroem, K.; Hagemann, U.B.; Cruciani, V.; Wengner, A.M.; Kristian, A.; Ellingsen, C.; Siemeister, G.; Bjerke, R.M.; Karlsson, J.; Ryan, O.B.; et al. Synergistic effect of mesothelin (MSLN)-TTC in combination with DNA damage response inhibitors. In Proceedings of the American Association for Cancer Research Annual Meeting 2018, Chicago, IL, USA, 14–18 April 2018; AACR: Philadelphia, PA, USA, 2018. [Google Scholar]
- Dondossola, E.; Casarin, S.; Paindelli, C.; De-Juan-Pardo, E.M.; Hutmacher, D.W.; Logothetis, C.J.; Friedl, P. Radium 223-mediated zonal cytotoxicity of prostate cancer in bone. J. Natl. Cancer Inst. 2019. [Google Scholar] [CrossRef]
- Bannik, K.; Zitzmann-Kolbe, S.; Scholz, A.; Jarke, S.; Jarzombek, M.; Sutter, A.; Siemeister, G.; Mumberg, D. Radium-223 α-particle radiation: Characterization of the in vitro effects on cancer cells in monotherapy and in combination with DNA repair inhibitors. In Proceedings of the American Association for Cancer Research Annual Meeting 2019, Atlanta, GA, USA, 29 March–3 April 2019; AACR: Philadelphia, PA, USA, 2019. [Google Scholar]
- Hagemann, U.B.; Zitzmann-Kolbe, S.; Kristian, A.; Sperl, C.; Schatz, C.A.; Bjerke, R.M.; Cuthbertson, A.S.; Hennekes, H.; Ziegelbauer, K.; Mumberg, D. MSLN-TTC (BAY 2287411) demonstrates increased activity in comparison to standard of care chemotherapy in models of acquired drug resistance. In Proceedings of the American Association for Cancer Research Annual Meeting 2019, Atlanta, GA, USA, 29 March–3 April 2019; AACR: Philadelphia, PA, USA, 2019. [Google Scholar]
- Hoskin, P.; Sartor, O.; O’Sullivan, J.M.; Johannessen, D.C.; Helle, S.I.; Logue, J.; Bottomley, D.; Nilsson, S.; Vogelzang, N.J.; Fang, F.; et al. Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: A prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Lancet Oncol. 2014, 15, 1397–1406. [Google Scholar] [CrossRef]
- Wissing, M.D.; van Leeuwen, F.W.; van der Pluijm, G.; Gelderblom, H. Radium-223 chloride: Extending life in prostate cancer patients by treating bone metastases. Clin. Cancer Res. 2013, 19, 5822–5827. [Google Scholar] [CrossRef]
- Coleman, R.; Aksnes, A.K.; Naume, B.; Garcia, C.; Jerusalem, G.; Piccart, M.; Vobecky, N.; Thuresson, M.; Flamen, P. A phase IIa, nonrandomized study of radium-223 dichloride in advanced breast cancer patients with bone-dominant disease. Breast Cancer Res. Treat. 2014, 145, 411–418. [Google Scholar] [CrossRef]
- Takalkar, A.; Adams, S.; Subbiah, V. Radium-223 dichloride bone-targeted alpha particle therapy for hormone-refractory breast cancer metastatic to bone. Exp. Hematol. Oncol. 2014, 3, 23. [Google Scholar] [CrossRef]
- Nilsson, S.; Larsen, R.H.; Fossa, S.D.; Balteskard, L.; Borch, K.W.; Westlin, J.E.; Salberg, G.; Bruland, O.S. First clinical experience with alpha-emitting radium-223 in the treatment of skeletal metastases. Clin. Cancer Res. 2005, 11, 4451–4459. [Google Scholar] [CrossRef]
- Nilsson, S.; Franzen, L.; Parker, C.; Tyrrell, C.; Blom, R.; Tennvall, J.; Lennernas, B.; Petersson, U.; Johannessen, D.C.; Sokal, M.; et al. Two-year survival follow-up of the randomized, double-blind, placebo-controlled phase II study of radium-223 chloride in patients with castration-resistant prostate cancer and bone metastases. Clin. Genitourin. Cancer 2013, 11, 20–26. [Google Scholar] [CrossRef]
- Elgqvist, J. Targeted alpha therapy: Part I. Curr. Radiopharm. 2011, 4, 176. [Google Scholar] [CrossRef]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fossa, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef]
- Morris, M.J.; Loriot, Y.; Sweeney, C.J.; Fizazi, K.; Ryan, C.J.; Shevrin, D.H.; Antonarakis, E.S.; Pandit-Taskar, N.; Deandreis, D.; Jacene, H.A.; et al. Radium-223 in combination with docetaxel in patients with castration-resistant prostate cancer and bone metastases: A phase 1 dose escalation/randomised phase 2a trial. Eur. J. Cancer 2019, 114, 107–116. [Google Scholar] [CrossRef]
- Soldatos, T.G.; Iakovou, I.; Sachpekidis, C. Retrospective Toxicological Profiling of Radium-223 Dichloride for the Treatment of Bone Metastases in Prostate Cancer Using Adverse Event Data. Medicina 2019, 55, 149. [Google Scholar] [CrossRef]
- Ciccia, A.; Elledge, S.J. The DNA damage response: Making it safe to play with knives. Mol. Cell 2010, 40, 179–204. [Google Scholar] [CrossRef]
- Bouwman, P.; Jonkers, J. The effects of deregulated DNA damage signalling on cancer chemotherapy response and resistance. Nat. Rev. Cancer 2012, 12, 587–598. [Google Scholar] [CrossRef]
- Hernandez, C.; Huebener, P.; Schwabe, R.F. Damage-associated molecular patterns in cancer: A double-edged sword. Oncogene 2016, 35, 5931–5941. [Google Scholar] [CrossRef]
- Wu, Q.; Allouch, A.; Martins, I.; Brenner, C.; Modjtahedi, N.; Deutsch, E.; Perfettini, J.L. Modulating Both Tumor Cell Death and Innate Immunity Is Essential for Improving Radiation Therapy Effectiveness. Front. Immunol. 2017, 8, 613. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Pestka, S.; Jubin, R.G.; Lyu, Y.L.; Tsai, Y.C.; Liu, L.F. Chemotherapeutics and radiation stimulate MHC class I expression through elevated interferon-beta signaling in breast cancer cells. PLoS ONE 2012, 7, e32542. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Schoenhals, J.E.; Li, A.; Valdecanas, D.R.; Ye, H.; Zang, F.; Tang, C.; Tang, M.; Liu, C.G.; Liu, X.; et al. Suppression of Type I IFN Signaling in Tumors Mediates Resistance to Anti-PD-1 Treatment That Can Be Overcome by Radiotherapy. Cancer Res. 2017, 77, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lora, A.; Martinez, M.; Algarra, I.; Gaforio, J.J.; Garrido, F. MHC class I-deficient metastatic tumor variants immunoselected by T lymphocytes originate from the coordinated downregulation of APM components. Int. J. Cancer 2003, 106, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, S.; Katz, S.C.; Shia, J.; Jarnagin, W.R.; Kingham, T.P.; Allen, P.J.; Fong, Y.; D’Angelica, M.I.; DeMatteo, R.P. Tumor MHC class I expression improves the prognostic value of T-cell density in resected colorectal liver metastases. Cancer Immunol. Res. 2014, 2, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Goeppert, B.; Frauenschuh, L.; Zucknick, M.; Roessler, S.; Mehrabi, A.; Hafezi, M.; Stenzinger, A.; Warth, A.; Pathil, A.; Renner, M.; et al. Major histocompatibility complex class I expression impacts on patient survival and type and density of immune cells in biliary tract cancer. Br. J. Cancer 2015, 113, 1343–1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reits, E.A.; Hodge, J.W.; Herberts, C.A.; Groothuis, T.A.; Chakraborty, M.; Wansley, E.K.; Camphausen, K.; Luiten, R.M.; de Ru, A.H.; Neijssen, J.; et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med. 2006, 203, 1259–1271. [Google Scholar] [CrossRef]
- Tai, Y.T.; Cho, S.F.; Anderson, K.C. Osteoclast Immunosuppressive Effects in Multiple Myeloma: Role of Programmed Cell Death Ligand 1. Front. Immunol. 2018, 9, 1822. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Ruiz, M.E.; Rodriguez, I.; Leaman, O.; Lopez-Campos, F.; Montero, A.; Conde, A.J.; Aristu, J.J.; Lara, P.; Calvo, F.M.; Melero, I. Immune mechanisms mediating abscopal effects in radioimmunotherapy. Pharmacol. Ther. 2019, 196, 195–203. [Google Scholar] [CrossRef]


| Authors | Year | Nonclinical Model | Main Findings | Ref |
|---|---|---|---|---|
| Henriksen et al. | 2002 | MT-1 human breast cancer bone metastasis model in rats | Radium-223 increases symptom-free survival. | [13] |
| Larsen et al. | 2006 | BALB/c mice | Doses ten-fold higher than therapeutic doses do not cause complete bone marrow suppression. | [77] |
| Suominen et al. | 2013 | MDA-MB-231 human breast cancer bone metastasis model in mice | Radium-223 prevents tumor-induced cachexia, decreases osteolysis, induces DNA DSBs, and extends survival alone and in combination with zoledronic acid or doxorubicin. | [15] |
| Malamas et al. | 2016 | In vitro | Radium-223 significantly enhances T cell-mediated lysis of prostate, breast, and lung carcinoma tumor cells by CD8+ cytotoxic T lymphocytes. | [78] |
| Suominen et al. | 2017 | 5TGM1 mouse myeloma model | Combination of radium-223 with bortezomib could constitute a novel, effective therapy for multiple myeloma. | [79] |
| Suominen et al. | 2017 | LNCaP, LuCaP 58 prostate cancer bone growth models in mice | Radium-223 inhibits tumor growth, tumor-induced osteoblastic bone growth, and protects normal bone architecture. Radium-223 shows a preferential uptake in bone lesions compared to normal bone and deposits in newly formed intratumoral bone matrix. Radium-223 induces DBS in local tumor cells, OBs and OCs. | [16] |
| Wengner et al. | 2018 | LNCaP human prostate cancer bone growth model in mice | Treatment with ATR inhibitor BAY 1895344 and radium-223 exhibits synergistic antitumor activity. | [80] |
| Wickstroem et al. | 2018 | OVCAR-3 ovarian cancer model in mice | MSLN-TTC increases the antitumor efficacy of ATR and PARP inhibitors. | [81] |
| Dondossola et al. | 2019 | PC3 and C4–2B human PCa cell lines in mouse bones | Micro-tumors showed good response to radium-223. Larger tumor areas were not as efficiently controlled by radium-223. | [82] |
| Bannik et al. | 2019 | In vitro | Synergistic in vitro effects were observed when radium-223 was combined with the ATR inhibitor BAY 1895344. | [83] |
| Hagemann et al. | 2019 | In vitro | MSLN-TTC is able to induce immunogenic cell death and secretion of pro-inflammatory cytokines in vitro. | [84] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suominen, M.I.; Wilson, T.; Käkönen, S.-M.; Scholz, A. The Mode-of-Action of Targeted Alpha Therapy Radium-223 as an Enabler for Novel Combinations to Treat Patients with Bone Metastasis. Int. J. Mol. Sci. 2019, 20, 3899. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20163899
Suominen MI, Wilson T, Käkönen S-M, Scholz A. The Mode-of-Action of Targeted Alpha Therapy Radium-223 as an Enabler for Novel Combinations to Treat Patients with Bone Metastasis. International Journal of Molecular Sciences. 2019; 20(16):3899. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20163899
Chicago/Turabian StyleSuominen, Mari I., Timothy Wilson, Sanna-Maria Käkönen, and Arne Scholz. 2019. "The Mode-of-Action of Targeted Alpha Therapy Radium-223 as an Enabler for Novel Combinations to Treat Patients with Bone Metastasis" International Journal of Molecular Sciences 20, no. 16: 3899. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20163899