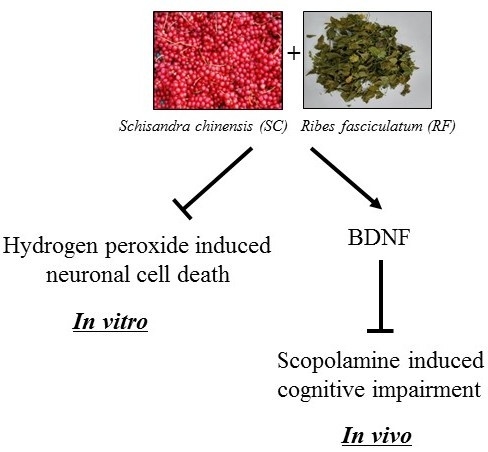
Synergistic Neuroprotective Effect of Schisandra chinensis and Ribes fasciculatum on Neuronal Cell Death and Scopolamine-Induced Cognitive Impairment in Rats
Abstract
:1. Introduction
2. Results
2.1. Verification of Increasing Hydrogen Peroxide (H2O2) Concentrations on Neuronal Cell Death
2.2. Protective Effect of SC and RF on H2O2-Induced Neuronal Cell Death
2.3. A Mixture of SC and RF Extracts Prevent Scopolamine-Induced Cognitive Impairment in Rats
2.4. A Mixture of SC and RF Extracts Exerts Neuroprotection via BDNF Signaling in Hippocampus
3. Discussion
4. Materials and Methods
4.1. Preparation of SC and RF Extracts
4.2. Cell Culture and Cytotoxicity Assay
4.3. In Vivo Experiments in Scopolamine-Treated Rats
4.4. Behavioral Studies
4.4.1. Passive Avoidance Test
4.4.2. Morris Water Maze
4.5. Protein Isolation and Western Blotting
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Knopman, D.S.; Petersen, R.C. Mild cognitive impairment and mild dementia: A clinical perspective. Mayo Clin. Proc. 2014, 89, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C.; Radhakrishnan, R. The prevention and treatment of cognitive decline and dementia: An overview of recent research on experimental treatments. Indian J. Psychiatry 2009, 51, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Kantarci, K.; Weigand, S.D.; Przybelski, S.A.; Shiung, M.M.; Whitwell, J.L.; Negash, S.; Knopman, D.S.; Boeve, B.F.; O’Brien, P.C.; Petersen, R.C.; et al. Risk of dementia in MCI: Combined effect of cerebrovascular disease, volumetric MRI, and 1H MRS. Neurology 2009, 72, 1519–1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cacabelos, R. Donepezil in Alzheimer’s disease: From conventional trials to pharmacogenetics. Neuropsychiatr. Dis. Treat. 2007, 3, 303–333. [Google Scholar] [PubMed]
- Onor, M.L.; Trevisiol, M.; Aguglia, E. Rivastigmine in the treatment of Alzheimer’s disease: An update. Clin. Interv. Aging 2007, 2, 17–32. [Google Scholar] [CrossRef] [PubMed]
- van Marum, R.J. Update on the use of memantine in Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2009, 5, 237–247. [Google Scholar] [CrossRef]
- Masuda, Y. Cardiac effect of cholinesterase inhibitors used in Alzheimer’s disease--from basic research to bedside. Curr. Alzheimer Res. 2004, 1, 315–321. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; He, X.; Liu, F.; Wang, J.; Feng, J. A review of polysaccharides from Schisandra chinensis and Schisandra sphenanthera: Properties, functions and applications. Carbohydr. Polym. 2018, 184, 178–190. [Google Scholar] [CrossRef]
- Giridharan, V.V.; Thandavarayan, R.A.; Sato, S.; Ko, K.M.; Konishi, T. Prevention of scopolamine-induced memory deficits by schisandrin B, an antioxidant lignan from Schisandra chinensis in mice. Free Radic. Res. 2011, 45, 950–958. [Google Scholar] [CrossRef]
- Song, J.X.; Lin, X.; Wong, R.N.; Sze, S.C.; Tong, Y.; Shaw, P.C.; Zhang, Y.B. Protective effects of dibenzocyclooctadiene lignans from Schisandra chinensis against beta-amyloid and homocysteine neurotoxicity in PC12 cells. Phytother. Res. 2011, 25, 435–443. [Google Scholar] [CrossRef]
- Han, Y.; Yang, H.; Li, L.; Du, X.; Sun, C. Schisanhenol improves learning and memory in scopolamine-treated mice by reducing acetylcholinesterase activity and attenuating oxidative damage through SIRT1-PGC-1alpha-Tau signaling pathway. Int. J. Neurosci. 2019, 129, 110–118. [Google Scholar] [CrossRef]
- Hu, D.; Li, C.; Han, N.; Miao, L.; Wang, D.; Liu, Z.; Wang, H.; Yin, J. Deoxyschizandrin isolated from the fruits of Schisandra chinensis ameliorates Abeta(1)(-)(4)(2)-induced memory impairment in mice. Planta Med. 2012, 78, 1332–1336. [Google Scholar]
- Hu, D.; Cao, Y.; He, R.; Han, N.; Liu, Z.; Miao, L.; Yin, J. Schizandrin, an antioxidant lignan from Schisandra chinensis, ameliorates Abeta1-42-induced memory impairment in mice. Oxid. Med. Cell. Longev. 2012, 2012, 721721. [Google Scholar] [CrossRef]
- Giridharan, V.V.; Thandavarayan, R.A.; Bhilwade, H.N.; Ko, K.M.; Watanabe, K.; Konishi, T. Schisandrin B, attenuates cisplatin-induced oxidative stress, genotoxicity and neurotoxicity through modulating NF-kappaB pathway in mice. Free Radic. Res. 2012, 46, 50–60. [Google Scholar] [CrossRef]
- Wei, B.B.; Liu, M.Y.; Chen, Z.X.; Wei, M.J. Schisandrin ameliorates cognitive impairment and attenuates Abeta deposition in APP/PS1 transgenic mice: Involvement of adjusting neurotransmitters and their metabolite changes in the brain. Acta Pharmacol. Sin. 2018, 39, 616–625. [Google Scholar] [CrossRef]
- Jeon, H.; Cha, D.S. Anti-aging properties of Ribes fasciculatum in Caenorhabditis elegans. Chin. J. Nat. Med. 2016, 14, 335–342. [Google Scholar]
- Dat, N.T.; Cai, X.F.; Shen, Q.; Lee, I.S.; Kim, Y.H. New inhibitor against nuclear factor of activated T cells transcription from Ribes fasciculatum var. chinense. Chem. Pharm. Bull. 2005, 53, 114–117. [Google Scholar] [CrossRef]
- Jung, J.W.; Kim, S.J.; Ahn, E.M.; Oh, S.R.; Lee, H.J.; Jeong, J.A.; Lee, J.Y. Ribes fasciculatum var. chinense Attenuated Allergic Inflammation In Vivo and In Vitro. Biomol. Ther. 2014, 22, 547–552. [Google Scholar] [Green Version]
- Park, E.J.; Ahn, J.J.; Kweon, J.H. Effect of Hot Water Extraction Conditions on Extraction and Antioxidant Properties of Freeze-Dried Schizandra chinensis Baillon. Korean J. Food Sci. Technol. 2013, 45, 550–556. [Google Scholar] [CrossRef]
- Ebert, U.; Kirch, W. Scopolamine model of dementia: Electroencephalogram findings and cognitive performance. Eur. J. Clin. Investig. 1998, 28, 944–949. [Google Scholar] [CrossRef]
- Cunha, C.; Brambilla, R.; Thomas, K.L. A simple role for BDNF in learning and memory? Front. Mol. Neurosci. 2010, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Cohen-Cory, S.; Kidane, A.H.; Shirkey, N.J.; Marshak, S. Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Dev. Neurobiol. 2010, 70, 271–288. [Google Scholar] [CrossRef] [Green Version]
- Gold, C.A.; Budson, A.E. Memory loss in Alzheimer’s disease: Implications for development of therapeutics. Expert Rev. Neurother. 2008, 8, 1879–1891. [Google Scholar] [CrossRef]
- Tarawneh, R.; Holtzman, D.M. The clinical problem of symptomatic Alzheimer disease and mild cognitive impairment. Cold Spring Harb. Perspect. Med. 2012, 2, a006148. [Google Scholar] [CrossRef]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef]
- Zhai, J.; Zhang, F.; Gao, S.; Chen, L.; Feng, G.; Yin, J.; Chen, W. Schisandra chinensis extract decreases chloroacetaldehyde production in rats and attenuates cyclophosphamide toxicity in liver, kidney and brain. J. Ethnopharmacol. 2018, 210, 223–231. [Google Scholar] [CrossRef]
- Nowak, A.; Zakłos-Szyda, M.; Błasiak, J.; Nowak, A.; Zhang, Z.; Zhang, B. Potential of Schisandra chinensis (Turcz.) Baill. in Human Health and Nutrition: A Review of Current Knowledge and Therapeutic Perspectives. Nutrients 2019, 11, 333. [Google Scholar] [Green Version]
- Zhang, M.; Xu, L.; Yang, H. Schisandra chinensis Fructus and Its Active Ingredients as Promising Resources for the Treatment of Neurological Diseases. Int. J. Mol. Sci. 2018, 19, 1970. [Google Scholar] [CrossRef]
- Li, C.L.; Tsuang, Y.H.; Tsai, T.H. Neuroprotective Effect of Schisandra Chinensis on Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Parkinsonian Syndrome in C57BL/6 Mice. Nutrients 2019, 11, 1671. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, C.; Xu, M.; Li, X.; Bi, K.; Jia, Y. Total Lignans of Schisandra chinensis Ameliorates Abeta1-42-Induced Neurodegeneration with Cognitive Impairment in Mice and Primary Mouse Neuronal Cells. PLoS ONE 2016, 11, e0152772. [Google Scholar]
- Haider, S.; Tabassum, S.; Perveen, T. Scopolamine-induced greater alterations in neurochemical profile and increased oxidative stress demonstrated a better model of dementia: A comparative study. Brain Res. Bull. 2016, 127, 234–247. [Google Scholar] [CrossRef]
- Umukoro, S.; Ugbomah, A.; Aderibigbe, A.; Omogbiya, A. Antioxidant Property of Jobelyn as the Possible Mechanism Underlying its Anti-amnesic Activity in Rodents. Basic Clin. Neurosci. 2013, 4, 42–49. [Google Scholar]
- Choi, S.H.; Woodlee, M.T.; Hong, J.J.; Schallert, T. A simple modification of the water maze test to enhance daily detection of spatial memory in rats and mice. J. Neurosci. Methods 2006, 156, 182–193. [Google Scholar] [CrossRef]
- Fayuk, D.; Yakel, J.L. Regulation of nicotinic acetylcholine receptor channel function by acetylcholinesterase inhibitors in rat hippocampal CA1 interneurons. Mol. Pharmacol. 2004, 66, 658–666. [Google Scholar] [CrossRef]
- Ohira, K.; Hayashi, M. A new aspect of the TrkB signaling pathway in neural plasticity. Curr. Neuropharmacol. 2009, 7, 276–285. [Google Scholar] [CrossRef]
- Song, M.; Martinowich, K.; Lee, F.S. BDNF at the synapse: Why location matters. Mol. Psychiatry 2017, 22, 1370–1375. [Google Scholar] [CrossRef]
- Hu, B.; Nikolakopoulou, A.M.; Cohen-Cory, S. BDNF stabilizes synapses and maintains the structural complexity of optic axons in vivo. Development 2005, 132, 4285–4298. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, A.L.; Matthews, B.J.; Meynard, M.M.; Hu, B.; Javed, S.; Cohen Cory, S. BDNF increases synapse density in dendrites of developing tectal neurons in vivo. Development 2006, 133, 2477–2486. [Google Scholar] [CrossRef] [Green Version]
- Yan, T.; He, B.; Wan, S.; Xu, M.; Yang, H.; Xiao, F.; Bi, K.; Jia, Y. Antidepressant-like effects and cognitive enhancement of Schisandra chinensis in chronic unpredictable mild stress mice and its related mechanism. Sci. Rep. 2017, 7, 6903. [Google Scholar] [CrossRef]
- Yan, T.; Xu, M.; Wan, S.; Wang, M.; Wu, B.; Xiao, F.; Bi, K.; Jia, Y. Schisandra chinensis produces the antidepressant-like effects in repeated corticosterone-induced mice via the BDNF/TrkB/CREB signaling pathway. Psychiatry Res. 2016, 243, 135–142. [Google Scholar] [CrossRef]
- Ahn, K. The worldwide trend of using botanical drugs and strategies for developing global drugs. BMB Rep. 2017, 50, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Amin, Z.A.; Abdulla, M.A.; Ali, H.M.; Alshawsh, M.A.; Qadir, S.W. Assessment of in vitro antioxidant, antibacterial and immune activation potentials of aqueous and ethanol extracts of Phyllanthus niruri. J. Sci. Food Agric. 2012, 92, 1874–1877. [Google Scholar] [CrossRef]
- Rush, D.K. Scopolamine amnesia of passive avoidance: A deficit of information acquisition. Behav. Neural Biol. 1988, 50, 255–274. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, E.; Ryu, M.J.; Kim, N.K.; Bae, M.H.; Seo, Y.; Kim, J.; Yeo, S.; Kanwal, M.; Choi, C.W.; Heo, J.Y.; et al. Synergistic Neuroprotective Effect of Schisandra chinensis and Ribes fasciculatum on Neuronal Cell Death and Scopolamine-Induced Cognitive Impairment in Rats. Int. J. Mol. Sci. 2019, 20, 4517. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20184517
Park E, Ryu MJ, Kim NK, Bae MH, Seo Y, Kim J, Yeo S, Kanwal M, Choi CW, Heo JY, et al. Synergistic Neuroprotective Effect of Schisandra chinensis and Ribes fasciculatum on Neuronal Cell Death and Scopolamine-Induced Cognitive Impairment in Rats. International Journal of Molecular Sciences. 2019; 20(18):4517. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20184517
Chicago/Turabian StylePark, Eunkuk, Min Jeong Ryu, Nam Ki Kim, Mun Hyoung Bae, Youngha Seo, Jeonghyun Kim, Subin Yeo, Memoona Kanwal, Chun Whan Choi, Jun Young Heo, and et al. 2019. "Synergistic Neuroprotective Effect of Schisandra chinensis and Ribes fasciculatum on Neuronal Cell Death and Scopolamine-Induced Cognitive Impairment in Rats" International Journal of Molecular Sciences 20, no. 18: 4517. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20184517