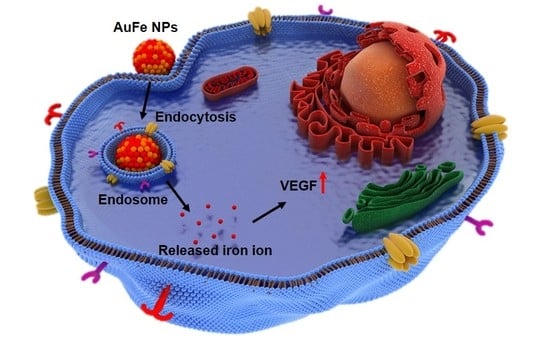
Enhancing the Wound Healing Effect of Conditioned Medium Collected from Mesenchymal Stem Cells with High Passage Number Using Bioreducible Nanoparticles
Abstract
:1. Introduction
2. Results
2.1. Characterization of pH-Sensitive AuFe NPs
2.2. Optimization of AuFe NP Concentration for the Treatment of hMSCs, Based on Cytotoxicity and VEGF Gene Expression
2.3. Effects of AuFe NPs on hMSCs In Vitro
2.4. Therapeutic Efficacy of CM Collected from hMSCs with AuFe NP Treatment
2.5. Improved Wound Healing Induced by CM Collected from AuFe NP-Treated hMSCs
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of AuFe NPs
4.3. Characterization
4.4. Fe Ion Release from the AuFe NPs under Acidic Condition
4.5. Cell Culture
4.6. Measurement of Cytotoxicity of AuFe NPs
4.7. qRT-PCR
4.8. RT-PCR
4.9. Intracellular Distribution of AuFe NPs
4.10. Wound Treatment
4.11. In Vivo Wound Closing Ratio
4.12. Histology
4.13. Immunohistochemistry
4.14. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Tang, Y.L.; Zhao, Q.; Zhang, Y.C.; Cheng, L.; Liu, M.; Shi, J.; Yang, Y.Z.; Pan, C.; Ge, J.; Phillips, M.I. Autologous mesenchymal stem cell transplantation induce VEGF and neovascularization in ischemic myocardium. Regul. Pept. 2004, 117, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.M.; Hur, S.M.; Park, K.Y.; Kim, C.K.; Kim, Y.M.; Kim, H.S.; Shin, H.C.; Won, M.H.; Ha, K.S.; Kwon, Y.G.; et al. Multiple paracrine factors secreted by mesenchymal stem cells contribute to angiogenesis. Vascul. Pharmacol. 2014, 63, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Tateishi-Yuyama, E.; Matsubara, H.; Murohara, T.; Ikeda, U.; Shintani, S.; Masaki, H.; Amano, K.; Kishimoto, Y.; Yoshimoto, K.; Akashi, H.; et al. Therapeutic angiogenesis using cell transplantation (TACT) study investigators therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: A pilot study and a randomised controlled trial. Lancet 2002, 360, 427–435. [Google Scholar] [CrossRef]
- Chen, J.S.; Wong, V.W.; Gurtner, G.C. Therapeutic potential of bone marrow-derived mesenchymal stem cells for cutaneous wound healing. Front. Immunol. 2012, 3, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, Y.; Liu, W.J.; Xue, P.; Ma, Y.; Zhang, L.Q.; Zhu, B.; Qi, M.; Li, L.Y.; Zhang, Y.J.; Wang, Q.T.; et al. Autophagy promotes MSC-mediated vascularization in cutaneous wound healing via regulation of VEGF secretion. Cell Death Dis. 2018, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, L.; Scott, P.G.; Tredget, E.E. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 2007, 25, 2648–2659. [Google Scholar] [CrossRef]
- Robey, T.E.; Saiget, M.K.; Reinecke, H.; Murry, C.E. Systems approaches to preventing transplanted cell death in cardiac repair. J. Mol. Cell. Cardiol. 2008, 45, 567–581. [Google Scholar] [CrossRef] [Green Version]
- Mottaghi, S.; Larijani, B.; Sharifi, A.M. Apelin 13: A novel approach to enhance efficacy of hypoxic preconditioned mesenchymal stem cells for cell therapy of diabetes. Med. Hypotheses 2012, 79, 717–718. [Google Scholar] [CrossRef]
- Abdelwahid, E.; Kalvelyte, A.; Stulpinas, A.; de Carvalho, K.A.T.; Guarita-Souza, L.C.; Foldes, G. Stem cell death and survival in heart regeneration and repair. Apoptosis 2015, 21, 252–268. [Google Scholar] [CrossRef] [Green Version]
- Hill, E.; Boontheekul, T.; Mooney, D.J. Regulating activation of transplanted cells controls tissue regeneration. Proc. Natl. Acad. Sci. USA 2006, 103, 2494–2499. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, N.Z.; Tuan, R.S. Regulation of stemness and stem cell niche of mesenchymal stem cells: Implications in tumorigenesis and metastasis. J. Cell. Physiol. 2010, 222, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Lazennec, G.; Jorgensen, C. Concise review: Adult multipotent stromal cells and cancer: Risk or benefit? Stem Cells 2008, 26, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Timmers, L.; Lim, S.K.; Hoefer, I.E.; Arslan, F.; Lai, R.C.; van Oorschot, A.A.; Goumans, M.J.; Strijder, C.; Sze, S.K.; Choo, A.; et al. Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res. 2011, 6, 206–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Xu, Y.; Zhao, J.; Zhang, Z.; Yang, R.; Xie, J.; Liu, X.; Qi, S. Correction: Conditioned medium from hypoxic bone marrow-derived mesenchymal stem cells enhances wound healing in mice. PLoS ONE 2014, 10, e0145565. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Lee, J.H.; Kim, H.J.; Park, M.K.; Huh, J.W.; Ro, J.Y.; Oh, Y.M.; Lee, S.D.; Lee, Y.S. Mesenchymal stem cell-conditioned media recovers lung fibroblasts from cigarette smoke-induced damage. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L891–L908. [Google Scholar] [CrossRef] [Green Version]
- Galderisi, U.; Helmbold, H.; Squillaro, T.; Alessio, N.; Komm, N.; Khadang, B.; Cipollaro, M.; Bohn, W.; Giordano, A. In vitro senescence of rat mesenchymal stem cells is accompanied by downregulation of stemness-related and DNA damage repair genes. Stem Cells Dev. 2009, 18, 1033–1042. [Google Scholar] [CrossRef]
- Crisostomo, P.R.; Wang, M.; Wairiuko, G.M.; Morrell, E.D.; Terrell, A.M.; Seshadri, P.; Nam, U.H.; Meldrum, D.R. High passage number of stem cells adversely affects stem cell activation and myocardial protection. Shock 2006, 26, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Sagaradze, G.; Grigorieva, O.; Nimiritsky, P.; Basalova, N.; Kalinina, N.; Akopyan, Z.; Efimenko, A. Conditioned medium from human mesenchymal stromal cells: Towards the clinical translation. Int. J. Mol. Sci. 2019, 20, 1656. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Nakashima, A.; Doi, S.; Ueno, T.; Okubo, T.; Kawano, K.; Kanawa, M.; Kato, Y.; Higashi, Y.; Masaki, T. Serum-free medium enhances the immunosuppressive and antifibrotic abilities of mesenchymal stem cells utilized in experimental renal fibrosis. Stem Cells Transl. Med. 2018, 7, 893–905. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.B.; Gray, K.M.; Santharam, Y.; Lamichhane, T.N.; Stroka, K.M.; Jay, S.M. Impact of cell culture parameters on production and vascularization bioactivity of mesenchymal stem cell-derived extracellular vesicles. Bioeng. Transl. Med. 2017, 2, 170–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.X.; Zeng, Z.C.; Sun, J.; Zeng, H.Y.; Huang, Y.; Zhang, Z.Y. Mesenchymal stem cell-conditioned medium prevents radiation-induced liver injury by inhibiting inflammation and protecting sinusoidal endothelial cells. J. Radiat. Res. 2015, 56, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wei, Z.; Li, B.; Li, J.; Lv, H.; Wu, L.; Zhang, H.; Yang, B.; Zhu, M.; Jiang, J. In vivo migration of Fe3O4@polydopamine nanoparticle-labeled mesenchymal stem cells to burn injury sites and their therapeutic effects in a rat model. Biomater. Sci. 2019, 7, 2861–2872. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, F.; Wang, Y.; Sun, X.; Choi, K.Y.; Liu, D.; Choi, J.S.; Shin, T.H.; Cheon, J.; Niu, G.; et al. Design considerations of iron-based nanoclusters for noninvasive tracking of mesenchymal stem cell homing. ACS Nano 2014, 8, 4403–4414. [Google Scholar] [CrossRef] [PubMed]
- Ejtehadifar, M.; Shamsasenjan, K.; Movassaghpour, A.; Akbarzadehlaleh, P.; Dehdilani, N.; Abbasi, P.; Molaeipour, Z.; Saleh, M. The effect of hypoxia on mesenchymal stem cell biology. Adv. Pharm. Bull. 2015, 5, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Lu, X.; Dong, W.; Yuan, Y.; Qian, J.; Li, Y.; Shi, J.; Liu, C. Endosomal pH-activatable magnetic nanoparticle-capped mesoporous silica for intracellular controlled release. J. Mater. Chem. 2013, 22, 15960. [Google Scholar] [CrossRef]
- Lee, G.W.; Seo, M.-S.; Kang, K.-K.; Oh, S.-K. Epidural fat-derived mesenchymal stem cell: First report of epidural fat-derived mesenchymal stem cell. Asian Spine J. 2019, 13, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Cárdenes, N.; Álvarez, D.; Sellarés, J.; Peng, Y.; Corey, C.; Wecht, S.; Nouraie, S.M.; Shanker, S.; Sembrat, J.; Bueno, M.; et al. Senescence of bone marrow-derived mesenchymal stem cells from patients with idiopathic pulmonary fibrosis. Stem Cell Res. Ther. 2018, 9, 257. [Google Scholar] [CrossRef]
- Proulx-Bonneau, S.; Guezguez, A.; Annabi, B.A. Concerted HIF-1α/MT1-MMP signalling axis regulates the expression of the 3BP2 adaptor protein in hypoxic mesenchymal stromal cells C. PLoS ONE 2011, 6, e21511. [Google Scholar] [CrossRef]
- Tsai, C.C.; Chen, Y.J.; Yew, T.L.; Chen, L.L.; Wang, J.Y.; Chiu, C.H.; Hung, S.C. Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down-regulation of E2A-p21 by HIF-TWIST. Blood 2011, 117, 459–469. [Google Scholar] [CrossRef] [Green Version]
- Qi, J.; Wang, J.J.; Duan, J.L.; Lu, Z.Y.; Yuan, Y.G. Leonurine Improves age-dependent impaired angiogenesis: Possible involvement of mitochondrial function and HIF-1α dependent VEGF activation. Front. Pharmacol. 2017, 8, 284. [Google Scholar] [CrossRef]
- Chang, E.I.; Loh, S.A.; Ceradini, D.J.; Chang, E.I.; Lin, S.E.; Bastidas, N.; Aarabi, S.; Chan, D.A.; Freedman, M.L.; Giaccia, A.J.; et al. Age decreases endothelial progenitor cell recruitment through decreases in hypoxia-inducible factor 1alpha stabilization during ischemia. Circulation 2007, 116, 2818–2829. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Li, W.; Liu, H.; Yin, D.; Zhao, J. The ROS/NF-κB/NR4A2 pathway is involved in H2O2 induced apoptosis of resident cardiac stem cells via autophagy. Oncotarget 2017, 8, 77634–77648. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, W.; Crisostomo, P.; Markel, T.; Meldrum, K.K.; Fu, X.Y.; Meldrum, D.R. STAT3 mediates bone marrow mesenchymal stem cell VEGF production. J. Mol. Cell. Cardiol. 2007, 42, 1009–1015. [Google Scholar] [CrossRef] [Green Version]
- Pons, J.; Huang, Y.; Arakawa-Hoyt, J.; Washko, D.; Takagawa, J.; Ye, J.; Grossman, W.; Su, H. VEGF improves survival of mesenchymal stem cells in infarcted hearts. Biochem. Biophys. Res. Commun. 2008, 376, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Geer, D.J.; Swartz, D.D.; Andreadis, S.T. Fibrin promotes migration in a three-dimensional in vitro model of wound regeneration. Tissue Eng. 2002, 8, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Kichenbrand, C.; Velot, E.; Menu, P.; Moby, V. Dental pulp stem cell-derived conditioned medium: An attractive alternative for regenerative therapy. Tissue Eng. Part. B Rev. 2018, 25, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Ashiba, K.; Terunuma, A.; Terunuma, H.; Takane, T.; Deng, X.; Yamashita, Y.; Watanabe, K. Immortalized mesenchymal stem cells producing conditioned medium in a large scale for therapeutic usage. Inflamm. Regen. 2015, 35, 057–060. [Google Scholar] [CrossRef] [Green Version]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef]
- Li, X.; Wei, Z.; Lv, H.; We, L.; Chu, Y.; Yao, H.; Li, J.; Zhang, H.; Yang, B.; Jiang, J. Iron oxide nanoparticles promote the migration of mesenchymal stem cells to injury sites. Int. J. Nanomed. 2019, 14, 573–589. [Google Scholar] [CrossRef]
- Bian, S.; Zhang, L.; Duan, L.; Wang, X.; Min, Y.; Yu, H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J. Mol. Med. 2013, 92, 387–397. [Google Scholar] [CrossRef]
- Desmouliere, A.; Darby, I.A.; Laverdet, B.; Bonté, F. Fibroblasts and myofibroblasts in wound healing. Clin. Cosmet. Investig. Dermatol. 2014, 7, 301–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faulknor, R.A.; Olekson, M.A.; Nativ, N.I.; Ghodbane, M.; Gray, A.J.; Berthiaume, F. Mesenchymal stromal cells reverse hypoxia-mediated suppression of α-smooth muscle actin expression in human dermal fibroblasts. Biochem. Biophys. Res. Commun. 2015, 458, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Lynam, E.C.; Xie, Y.; Dawson, R.; Mcgovern, J.; Upton, Z.; Wang, X. Severe hypoxia and malnutrition collectively contribute to scar fibroblast inhibition and cell apoptosis. Wound Repair Regen. 2015, 23, 664–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saucedo, L.J.; Gao, X.; Chiarelli, D.A.; Li, L.; Pan, D.; Edgar, B.A. Rheb promotes cell growth as a component of the insulin/TOR signalling network. Nat. Cell. Biol. 2003, 5, 566–571. [Google Scholar] [CrossRef] [PubMed]





| Primer | Sequence |
|---|---|
| Human GAPDH | F: 5′-GTC GGA GTC AAC GGA TTT GG-3′ R: 5′-GGG TGG AAT CA TTG GAA CAT-3′ |
| Human VEGF | F: 5′-GAG GGC AGA ATC ATC ACG AAG T-3′ R: 5′-CAC CAG GGT CTC GAT TGG AT-3′ |
| Mouse CD31 | F: 5′-CAA ACA GAA ACC CGT GGA GAT G-3′ R: 5′-ACC GTA ATG GCT GTT GGC TTC-3′ |
| Mouse SM-α | F: 5′-CAG GCA TGG ATG GCA TCA ATC AC-3′ R: 5′-ACT CTA GCT GTG AAG TCA GTG TCG-3′ |
| Primer | Sequence |
|---|---|
| Human β-actin | F: 5′-GCA CTC TTC CAG CCT TCC TTC C-3′ R: 5′-TCA CCT TCA CCG TTC CAG TTT TT-3′ |
| Human VEGF | F: 5′-GCA GAA GGA GGA GGG CAG AAT-3′ R: 5′-ACA CTC CAG GCC CTC GTC ATT-3′ |
| Human HIF-1α | F: 5′-TAT GAC CTG CTT GGT GCT GA-3′ R: 5′-GGG AGA AAA TCA AGT CGT GC-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Im, G.-B.; Kim, Y.H.; Kim, Y.-J.; Kim, S.-W.; Jung, E.; Jeong, G.-J.; Wang, K.; Kim, J.; Kim, D.-I.; Kim, T.-H.; et al. Enhancing the Wound Healing Effect of Conditioned Medium Collected from Mesenchymal Stem Cells with High Passage Number Using Bioreducible Nanoparticles. Int. J. Mol. Sci. 2019, 20, 4835. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20194835
Im G-B, Kim YH, Kim Y-J, Kim S-W, Jung E, Jeong G-J, Wang K, Kim J, Kim D-I, Kim T-H, et al. Enhancing the Wound Healing Effect of Conditioned Medium Collected from Mesenchymal Stem Cells with High Passage Number Using Bioreducible Nanoparticles. International Journal of Molecular Sciences. 2019; 20(19):4835. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20194835
Chicago/Turabian StyleIm, Gwang-Bum, Yeong Hwan Kim, Yu-Jin Kim, Sung-Won Kim, Euiyoung Jung, Gun-Jae Jeong, Ke Wang, Jinheung Kim, Dong-Ik Kim, Tae-Hyung Kim, and et al. 2019. "Enhancing the Wound Healing Effect of Conditioned Medium Collected from Mesenchymal Stem Cells with High Passage Number Using Bioreducible Nanoparticles" International Journal of Molecular Sciences 20, no. 19: 4835. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20194835