Phytohormones (Auxin, Gibberellin) and ACC Deaminase In Vitro Synthesized by the Mycoparasitic Trichoderma DEMTkZ3A0 Strain and Changes in the Level of Auxin and Plant Resistance Markers in Wheat Seedlings Inoculated with this Strain Conidia
Abstract
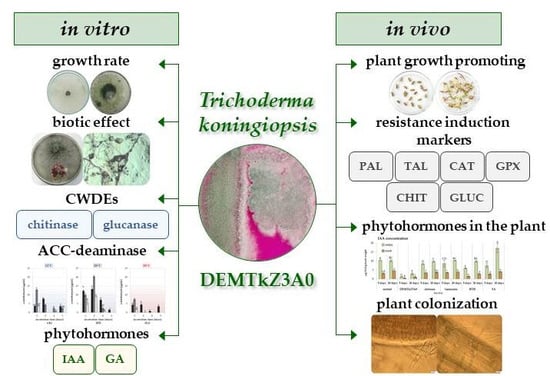
:1. Introduction
2. Results
2.1. Determination of Phylogenetic Position of DEMTkZ3A0 Isolate
2.2. Growth Rate of Trichoderma DEMTkZ3A0 Strain Under Various Temperature and Carbon Conditions
2.3. Mycoparasitic Effect of Trichoderma DEMTkZ3A0 Strain on Phytopathogens
2.4. Chitinolytic and Glucanolytic Activities in Culture Supernatants of Trichoderma Strain Grown in Media Containing Glucose, Chitin and Lyophilized Cell Wall of Phytopathogenic F. culmorum Strain
2.5. Study of the Synthesis of IAA, GA and ACC-Deaminase by Trichoderma DEMTkZ3A0 Strain Under Various Temperature Conditions and the Presence of Amino Acid Precursors
Principal Component Analysis (PCA) for the Physical of the Trichoderma DEMTkZ3A0 Strain Growing Parameters and IAA, GA Concentration, and ACC-Deaminase Activity
2.6. Promotion of Winter Wheat Seeds Growth and Activity of Plant Resistance Induction Markers
Principal Component Analysis (PCA) for Activity of Plant Resistance Markers, IAA, and TPC Concentration in Wheat
3. Discussion
4. Materials and Methods
4.1. PCR Amplification and Sequencing of the Fungal ITS Region
4.2. Fungal Isolates and Their Molecular Identification
4.3. Storage Conditions of Trichoderma DEMTkZ3A0 and Fusarium spp. Strains
4.4. Growth Rate of Trichoderma DEMTkZ3A0 Strain
4.5. Biotic Effect and Mycoparasitic Interaction Between Trichoderma DEMTkZ3A0 Strain and Phytopathogens
4.6. Preparation of Trichoderma DEMTkZ3A0 Strain Conidia
4.7. Study of the Chitinolytic and Glucanolytic Activity in Trichoderma DEMTkZ3A0 Strain Liquid Culture
4.8. Study of the Synthesis of IAA, GA and ACC-deaminase in Trichoderma DEMTkZ3A0 Strain Liquid Culture
4.8.1. Determination of IAA Concentration
4.8.2. Determination of GA Concentration
4.8.3. Determination of ACC-Deaminase Activity
4.9. In Vivo Stimulation of Wheat Growth and Analysis of the Activity of Plant Resistance Markers
4.9.1. Preparation of Seeds
4.9.2. Study of Root Colonization by Trichoderma DEMTkZ3A0 Strain and Wheat Growth Promotion
4.9.3. Enzyme Extraction
4.9.4. Phenylalanine Ammonia Lyase (PAL) Assay
4.9.5. Tyrosine Phenol-Lyase (TAL) Assay
4.9.6. Guaiacolic Peroxidase (GPX) Assay
4.9.7. Catalase (CAT) Assay
4.9.8. Chitinase (CHIT) and Glucanase (GLUC) Assay
4.9.9. Determination of Total Phenolic Compounds Content in Stems and Roots of Wheat
4.10. Statistical Analysis
5. Conclusions and Future Perspective
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| IAA | indoleacetic acid |
| GA | gibberellic acid |
| ACC | 1-aminocyclopropane-1-carboxylate |
| RBCs | root border cells |
| PAL | phenylalanine ammonia lyase |
| TAL | tyrosine ammonia lyase |
| CAT | catalase |
| GPX | guaiacol peroxidase |
| GLUC | glucanase |
| CHIT | chitinase |
| GAs | gibberellins |
| ABA | abscisic acid |
| CWDE | cell wall degrading enzymes |
| FHB | Fusarium head blight |
| PGPR | plant growth promoting rhizobacteria |
| CW | cell wall |
| ROS | reactive oxygene species |
| JA | jasmonic acid |
| PAMP | pathogen-associated molecular pattern |
| PTI | (PAMP)-tiggered immune signaling pathways |
| ETI | effector-tiggered immunity |
| SAR | systemic acquired resistance |
| MAMPs | microbe-associated molecular patterns |
| DAMPs | damage-associated molecular patterns |
| PRRs | plasma membrane-localized receptors |
| SA | salicylic acid |
| PR | pathogenesis-related protein |
| PGPF | plant growth-promoting fungi |
| SOD | superoxide dismutase |
| DRMO | deleterious rhizosphere microorganisms |
| PDA | potato dextrose agar |
| RB medium | Ryes Byrde medium |
| ACCD | ACC-deaminase activity |
References
- Shi, W.L.; Chen, X.L.; Wang, L.X.; Gong, Z.T.; Li, S.; Li, C.L.; Xie, B.B.; Zhang, W.; Shi, M.; Li, C.; et al. Cellular and molecular insight into the inhibition of primary root growth of Arabidopsis induced by peptaibols, a class of linear peptide antibiotics mainly produced by Trichoderma spp. J. Exp. Bot. 2016, 67, 2191–2205. [Google Scholar] [CrossRef] [PubMed]
- Chanclud, E.; Lacombe, B. Plant hormones: key players in gut microbiota and human diseases? Trends Plant Sci. 2017, 22, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Jaroszuk-Ściseł, J.; Kurek, E.; Trytek, M. Efficiency of indoleacetic acid, gibberellic acid and ethylene synthesized in vitro by Fusarium culmorum strains with different effects on cereal growth. Biologia 2014, 69, 281–292. [Google Scholar] [CrossRef]
- Mefteh, F.B.; Daoud, A.; Bouket, A.C.; Alenezi, F.N.; Luptakova, L.; Rateb, M.E.; Kadri, A.; Gharsallah, N.; Belbahri, L. Fungal root microbiome from healthy and brittle leaf diseased date palm trees (Phoenix dactylifera L.) reveals a hidden untapped arsenal of antibacterial and broad spectrum antifungal secondary metabolites. Front. Microbiol. 2017, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Tsavkelova, E.A.; Klimova, S.Y.; Chedyntseva, T.A.; Netrusov, A.I. Microbial producers of plant growth stimulators and their practical use: A review. Appl. Biochem. Micro. 2006, 42, 117–126. [Google Scholar] [CrossRef]
- Druzhinina, I.S.; Seidl-Seiboth, V.; Herrera-Estrella, A.; Horwitz, B.A.; Kenerley, C.M.; Monte, E.; Mukherjee, P.K.; Zeilinger, S.; Grigoriev, I.V.; Kubicek, C.P. Trichoderma: the genomics of opportunistic success. Nat. Microbiol. Rev. 2011, 16, 749–759. [Google Scholar] [CrossRef]
- Klein, E.; Eveleigh, D.E. Ecology of Trichoderma. In Trichoderma and Gliocladium, Basic Biology, Taxonomy and Genetics; Kubicek, C.P., Harman, G.E., Eds.; Taylor & Francis: London, UK, 1998; Volume 1, pp. 57–74. [Google Scholar]
- Druzhinina, I.S.; Komoń-Zelazowska, M.; Ismaiel, A.; Jaklitsch, W.; Mullawa, T.; Samuels, G.J.; Kubicek, C.P. Molecular phylogeny and species delimitation in the section Longibrachiatum of Trichoderma. Fungal Genet. Biol. 2012, 49, 358–368. [Google Scholar] [CrossRef]
- Hassan, M.M.; Farid, M.A.; Gaber, A. Rapid identification of Trichoderma koningiopsis and Trichoderma longibrachiatum using sequence-characterized amplified region markers. Egypt J. Biol. Pest. Control 2019, 29, 13. [Google Scholar] [CrossRef]
- Samuels, G.J.; Dodd, S.L.; Lu, B.S.; Petrini, O.; Schroers, H.J.; Druzhinina, I.S. The Trichoderma koningii aggregate species. Stud. Mycol. 2006, 56, 67–133. [Google Scholar] [CrossRef]
- Bissett, J.; Gams, W.; Jaklitsch, W.; Samuels, G.J. Accepted Trichoderma names in the year 2015. IMA Fungus 2015, 6, 263–295. [Google Scholar] [CrossRef]
- Ramirez, C.A.; Kloepper, J.W. Plant growth promotion by Bacillus amyloliquefaciens FZB45 depends on inoculum rate and P-related soil properties. Biol. Fertil. Soils 2010, 46, 835–844. [Google Scholar] [CrossRef]
- Zhang, F.; Yuan, J.; Yang, X.; Cui, X.; Chen, L.; Ran, W.; Shen, Q. Putative Trichoderma harzianum mutant promotes cucumber growth by enhanced production of indole acetic acid and plant colonization. Plant Soil 2013, 368, 433–444. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, Z.; Yang, X.; Shen, Q. Trichoderma harzianum T-E5 significantly affects cucumber root exudates and fungal community in the cucumber rhizosphere. Appl. Soil Ecol. 2013, 72, 41–48. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Yaqian, L.; Yu, C.; Wang, Q.-Q.; Wang, M.; Sun, J.; Gao, J.-X.; Chen, J. Synergistic effect of Trichoderma-derived antifungal metabolites and cell wall degrading enzymes on enhanced biocontrol of Fusarium oxysporum f. sp. cucumerinum. Biol. Control 2016, 94, 37–46. [Google Scholar] [CrossRef]
- Gruber, S.; Seidl-Seiboth, V. Self versus non-self: fungal cell wall degradation in Trichoderma. Microbiology 2012, 158, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Gajera, H.; Rinkal, D.; Sunil, P.; Mansukh, K.; Balubhai, G. Molecular mechanism of Trichoderma as bio-control agents against phytopathogen system—a review. Curr. Res. Microbiol. Biotechnol. 2013, 1, 133–142. [Google Scholar]
- Zeilfinger, S.; Gruber, S.; Bansal, R.; Mukherjee, P.K. Secondary metabolism in Trichoderma—Chemistry meets genomics. Fungal Biol. Rev. 2016, 30, 74–90. [Google Scholar] [CrossRef]
- Lu, Z.; Tombolini, R.; Woo, S.; Zeilinger, S.; Lorito, M.; Jansson, J.K. In vivo study of Trichoderma-pathogen-plant interactions, using constitutive and inducible green fluorescent protein reporter Systems. Appl. Environ. Micro. 2004, 70, 3073–3081. [Google Scholar] [CrossRef]
- Gajera, H.P.; Bambharolia, R.P.; Patel, S.V.; Khatrani, T.J.; Goalkiya, B.A. Antagonism of Trichoderma spp. against Macrophomina phaseolina: Evaluation of coiling and cell wall degrading enzymatic activities. Plant Pathol. Microb. 2012, 3, 7. [Google Scholar] [CrossRef]
- Zachow, C.; Berg, G. Understanding the diversity and versatility of Trichoderma by next-generation sequencing. In Biotechnology and Biology of Trichoderma; Elsevier: Amsterdam, The Netherlands, 2014; pp. 57–65. [Google Scholar] [CrossRef]
- Sharma, M.; Guleria, S.; Singh, K.; Chauhan, A.; Kulshrestha, S. Mycovirus associated hypovirulence, a potential method for biological control of Fusarium species. Virusdisease 2018, 29, 134–140. [Google Scholar] [CrossRef]
- Jain, S.K.; Khilari, K.; Ali, M.; Singh, R. Response of Fusarium monoliforme—The causal organism of Bakanae Disease of Rice against different fungicides. Bioscan 2014, 9, 413–416. [Google Scholar]
- Lin, Z.; Xu, S.; Que, Y.; Wang, J.; Comstock, J.C. Species-specific detection and identification of Fusarium species complex, the causal agent of Sugarcane PokkahBoeng in China. PLoS ONE 2014, 9, e104195. [Google Scholar] [CrossRef]
- Rusli, M.H.; Idris, A.S.; Cooper, R.M. Evaluation of Malaysian oil palm progenies for susceptibility, resistance or tolerance of Fusarium oxysporum f. sp. elaeidis and defense-related gene expression in roots. Plant Pathol. 2015, 64, 638–647. [Google Scholar] [CrossRef]
- Supyani, S.; Widadi, S. Hypovirulent isolates of Fusarium collected from chili crops in Boyolali Regency, Central Java, Indonesia. Agrivita 2015, 37, 67–74. [Google Scholar] [CrossRef]
- Vishwakarma, S.K.; Kumar, P.; Nigam, A.; Singh, A.; Kumar, A. Pokkah Boeng: An emerging disease of sugarcane. J. Plant Pathol. Microbiol. 2013, 4, 170. [Google Scholar] [CrossRef]
- Zhang, H.; Mallik, A.; Zeng, R.S. Control of panama disease of banana by rotating and intercropping with Chinese chive (Allium tuberosum) role of plant volatiles. J. Chem. Ecol. 2013, 39, 243–252. [Google Scholar] [CrossRef]
- Dean, R.A.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.G.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [Green Version]
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef]
- Riad, E.M.R.S.; Zeidan, E.S.H. First record of core rot on apple fruit cv. anna 106 local cultivar in Egypt. J. Agric. Technol. 2015, 11, 1371–1380. [Google Scholar]
- Munkvold, G.P. Fusarium species and their associated mycotoxins. Methods Mol. Biol. 2017, 1542, 51–106. [Google Scholar] [CrossRef]
- Sadfi, N.; Cherif, M.; Hajlaoui, M.R.; Boudabbous, A. Biological control of the potato tubers dry rot caused by Fusarium roseum var. sambucinum under greenhouse, field and storage conditions using Bacillus spp. isolates. J. Phytopathol. 2002, 150, 640–648. [Google Scholar] [CrossRef]
- Kurek, E.; Jaroszuk-Ściseł, J. Rye (Secale cereale) growth promotion by Pseudomonas fluorescens strains and their interactions with Fusarium culmorum under various soil conditions. Biol. Control 2003, 26, 448–456. [Google Scholar] [CrossRef]
- Recep, K.; Fikrettin, S.; Erkol, D.; Cafer, E. Biological control of the potato dry rot caused by Fusarium species using PGPR strains. Biol. Control 2009, 50, 194–198. [Google Scholar] [CrossRef]
- Wachowska, U.; Kucharska, K.; Jedryczka, M.; Lobik, N. Microorganisms as biological control agents against Fusarium pathogens in winter wheat. Pol. J. Environ. Stud. 2013, 22, 591–597. [Google Scholar]
- Fravel, D.; Olivain, C.; Alabouvette, C. Research review Fusarium oxysporum and its biocontrol. New Phytol. 2003, 157, 493–502. [Google Scholar] [CrossRef]
- Jaroszuk-Ściseł, J.; Kurek, E.; Winiarczyk, K.; Baturo, A.; Łukanowski, A. Colonization of root tissues and protection against fusarium wilt of rye (Secale cereale) by nonpathogenic rhizosphere strains of Fusarium culmorum. Biol. Control. 2008, 45, 297–307. [Google Scholar] [CrossRef]
- Verma, M.; Brar, S.; Tyagi, R.; Surampalli, R.; Valero, J. Antagonistic fungi, Trichoderma spp.: Panoply of biological control. Biochem. Eng. J. 2007, 37, 1–20. [Google Scholar] [CrossRef]
- Poosapati, S.; Ravulapalli, P.D.; Tippirishetty, N.; Vishwanathaswamy, D.K.; Chunduri, S. Selection of high temperature and salinity tolerant Trichoderma isolates with antagonistic activity against Sclerotium rolfsii. Springer Plus. 2014, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species - opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Shakeri, J.; Foster, H.A. Proteolytic activity and antibiotic production by Trichoderma harzianum in relation to pathogenicity to insects. Enzym. Microb. Tech. 2007, 40, 961–968. [Google Scholar] [CrossRef]
- Jeger, M.J.; Jeffries, P.; Elad, Y.; Xu, X.M. A generic theoretical model for biological control of foliar plant diseases. J. Theor. Biol. 2009, 256, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Segarra, G.; Casanova, E.; Bellido, D.; Odena, M.A.; Oliveira, E.; Trillas, I. Proteome, salicylic acid, and jasmonic acid changes in cucumber plants inoculated with Trichoderma asperellum strain T34. Proteomics 2007, 7, 3943–3952. [Google Scholar] [CrossRef] [PubMed]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, D.L.; Marra, R.; Woo, S.L.; Lorito, M. Trichoderma–plant–pathogen interactions. Soil Biol. Biochem. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- Ownley, B.H.; Gwinn, K.D.; Vega, F.E. Endophytic fungal entomopathogens with activity against plant pathogens: Ecology and evolution. BioControl 2010, 55, 113–128. [Google Scholar] [CrossRef]
- Schulz, B.; Boyle, C. The endophytic continuum. Mycol. Res. 2005, 109, 661–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conrath, U.; Beckers, G.J.M.; Flors, V.; García-Agustín, P.; Jakab, G.; Mauch, F.; Newman, M.A.; Pieterse, C.M.J.; Poinssot, B.; Pozo, M.J.; et al. Priming: Getting ready for battle. Mol. Plant-Microbe Interact. 2006, 19, 1062–1071. [Google Scholar] [CrossRef]
- Van Wees, S.C.M.; Van der Ent, S.; Pieterse, C.M.J. Plant immune responses triggered by beneficial microbes. Curr. Opin. Plant Biol. 2008, 11, 443–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyde, K.D.; Soytong, K. The fungal endophyte dilemma. Fungal Divers. 2008, 33, 163–173. [Google Scholar]
- Partida-Martinez, L.P.; Heil, M. The microbe-free plant: Fact or artifact? Front. Plant Sci. 2011, 2, 1–16. [Google Scholar] [CrossRef]
- Perazzolli, M.; Dagostin, S.; Ferrar, A.; Elad, Y.; Pertot, I. Induction of systemic resistance against Plasmopara viticola in grapevine by Trichoderma harzianum T39 and benzothiadiazole. Biol. Control 2008, 47, 228–234. [Google Scholar] [CrossRef]
- Korolev, N.; David, D.R.; Elad, Y. The role of phytohormones in basal resistance and Trichoderma-induced systemic resistance to Botrytis cinerea in Arabidopsis thaliana. BioControl 2008, 53, 667–683. [Google Scholar] [CrossRef]
- Ryu, C.M.; Farag, M.A.; Hu, C.H.; Reddy, M.S.; Kloepper, J.W.; Pare, P.W. Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol. 2004, 134, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.C.; Bakker, P.A.H.M.; Pieterse, C.M.J. Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol. 1998, 36, 453–483. [Google Scholar] [CrossRef] [PubMed]
- Jaroszuk-Ściseł, J.; Kurek, E.; Słomka, A.; Janczarek, M.; Rodzik, B. Activities of cell wall degrading enzymes in autolyzing cultures of three Fusarium culmorum isolates: Growth promoting, deleterious and pathogenic to rye (Secale cereale). Mycologia 2011, 103, 929–945. [Google Scholar] [CrossRef]
- Jaroszuk-Ściseł, J.; Kurek, E. Hydrolysis of fungal and plant cell walls by enzymatic complexes from cultures of Fusarium isolates with different aggressiveness to rye (Secale cereale). Arch. Microbiol. 2012, 194, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Vallad, G.E.; Goodman, R.M. Systemic acquired resistance and induced systemic resistance in conventional agriculture. Crop. Sci. 2004, 44, 1920–1934. [Google Scholar] [CrossRef]
- Chen, L.H.; Yang, X.M.; Raza, W.; Li, J.H.; Liu, Y.X.; Qiu, M.H.; Zhang, F.G.; Shen, Q.R. Trichoderma harzianum SQR-T037 rapidly degrades allelochemicals in rhizospheres of continuously cropped cucumbers. Appl. Microbiol. Biotechnol. 2011, 89, 1653–1663. [Google Scholar] [CrossRef]
- Thilagavathi, R.; Saravanakumar, D.; Ragupathi, N.; Samiyappan, R. A combination of biocontrol agents improves the management of dry root rot (Macrophomina phaseolina) in greengram. Phytopathol. Mediterr. 2007, 46, 157–167. [Google Scholar] [CrossRef]
- Brunetti, C.; Fini, A.; Sebastiani, F.; Gori, A.; Tattini, M. Modulation of phytohormone signaling: A primary function of flavonoids in plant–environment interactions. Front. Plant Sci. 2018, 9, 1042. [Google Scholar] [CrossRef]
- Gutjahr, C.; Paszkowski, U. Weights in the balance: jasmonic acid and salicylic acid signaling in root-biotroph interactions. Mol. Plant Microbe Interact. 2009, 22, 763–772. [Google Scholar] [CrossRef]
- Vandenkoornhuyse, P.; Quaiser, A.; Duhamel, M.; Le Van, A.; Dufresne, A. The importance of the microbiome of the plant holobiont. New Phytol. 2015, 206, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Boller, T.; Felix, G. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 2009, 60, 379–406. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, H.; Tsuda, K.; Parker, J.E. Effector-triggered immunity: from pathogen perception to robust defense. Annu. Rev. Plant Biol. 2015, 66, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Feng, B.; He, P.; Shan, L. From chaos to harmony: responses and signaling upon microbial pattern recognition. Annu. Rev. Phytopathol. 2017, 55, 109–137. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.Q.; Dong, X. Systemic acquired resistance: turning local infection into global defense. Annu. Rev. Plant Biol. 2013, 64, 839–863. [Google Scholar] [CrossRef]
- Hou, S.; Liu, Z.; Shen, H.; Wu, D. Damage-associated molecular pattern-triggered immunity in plants. Front. Plant Sci. 2019, 10, 646. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.J.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C.M. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef]
- Shigenaga, A.; Berens, M.M.L.; Tsuda, K.; Argueso, C.T. Towards engineering of hormonal crosstalk in plant immunity. Curr. Opin. Plant Biol. 2017, 38, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Gimenez-Ibanez, S.; Solano, R. Nuclear jasmonate and salicylate signalling and crosstalk in defense against pathogens. Front. Plant Sci. 2013, 4, 1–11. [Google Scholar] [CrossRef]
- Sels, J.; Mathys, J.; De Coninck, B.M.; Cammue, B.P.; De Bolle, M.F. Plant pathogenesis-related (PR) proteins: a focus on PR peptides. Plant Physiol. Biochem. 2008, 46, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Breen, S.; Williams, S.J.; Outram, M.; Kobe, B.; Solomon, P.S. Emerging insights into the functions of pathogenesis-related protein 1. Trends Plant Sci. 2017, 22, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Ganai, B.A.; Kamili, A.Z.; Bhat, A.A.; Mir, Z.A.; Bhat, J.A.; Tyagi, A.; Islam, S.T.; Mushtaq, M.; Yadav, P.; et al. Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiol. Res. 2018, 212–213, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.R.; Jones, J.D. Hormone (dis)harmony moulds plant health and disease. Science 2009, 324, 750–752. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.J.; Leon-Reyes, A.; Van der Ent, S.; Van Wees, S.C.M. Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 2009, 5, 308–316. [Google Scholar] [CrossRef] [Green Version]
- Glick, B.R.; Penrose, D.M.; Li, J. A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J. Theor Biol. 1998, 190, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Modulation of plant ethylene levels by the bacterial enzyme ACC deaminase. FEMS Microbiol Lett. 2005, 1–7. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, C.; Zhang, F.; Gao, L.; Liu, J.; Chen, L.; Fan, X.; Liu, C.; Zhang, K.; Hea, Y.; et al. Trichoderma harzianum containing 1-aminocyclopropane-1-carboxylate deaminase and chitinase improved growth and diminished adverse effect caused by Fusarium oxysporumin soybean. J. Plant Physiol. 2017, 210, 84–94. [Google Scholar] [CrossRef]
- Ozimek, E.; Jaroszuk-Ściseł, J.; Bohacz, J.; Korniłłowicz-Kowalska, T.; Tyśkiewicz, R.; Słomka, A.; Nowak, A.; Hanaka, A. Synthesis of indoleacetic acid, gibberellic acid and ACC-deaminase by Mortierella strains promote winter wheat seedlings growth under different conditions. Int. J. Mol. Sci. 2018, 19, 3218. [Google Scholar] [CrossRef]
- Kredics, L.; Antal, Z.; Manczinger, L.; Szekeres, A.; Kevei, F.; Nag, E. Influence of environmental parameters on Trichoderma strains with biocontrol potential. Food Technol. Biotechnol. 2003, 41, 37–42. [Google Scholar]
- Danielson, R.M.; Davey, C.B. The abundance of Trichoderma propagules and the distribution of species in forest soils. Soil Biol. Biochem. 1973, 5, 495–504. [Google Scholar] [CrossRef]
- Samuels, G.J. Trichoderma: A review of biology and systematics of the genus. Mycol. Res. 1996, 100, 923–935. [Google Scholar] [CrossRef]
- Eastburn, D.M.; Butler, E.E. Effect of soil moisture and temperature on thesaprophytic ability of Trichoderma harzianum. Mycologia 1991, 83, 257–263. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Jukes, T.H.; Cantor, C.R. Evolution of protein molecules. In Mammalian Protein Metabolism; Munro, H.N., Ed.; Academic Press: New York, NY, USA, 1969; pp. 21–132. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for a bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Reyes, F.; Byrde, R.J.W. Partial purification and properties of a β-N-acetylglucosaminidase from the fungus Sclerotinia fructigena. Biochem. J. 1973, 131, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Jaroszuk-Ściseł, J.; Kurek, E.; Rodzik, B.; Winiarczyk, K. Interactions between rye (Secale cereale) root border cells (RBCs) and pathogenic and nonpathogenic rhizosphere strains of Fusarium culmorum. Mycol. Res. 2009, 113, 1053–1061. [Google Scholar] [CrossRef]
- Hanaka, A.; Nowak, A.; Plak, A.; Dresler, S.; Ozimek, E.; Jaroszuk-Ściseł, J.; Wójciak-Kosior, M.; Sowa, M. Bacterial isolate inhabiting Spitsbergen soil modifies the physiological response of Phaseolus coccineus in control conditions and under exogenous application of methyl jasmonate and copper excess. Int. J. Mol. Sci. 2019, 20, 1909. [Google Scholar] [CrossRef]
- Schlaeppi, K.; Dombrowski, N.; Oter, R.G.; Van Themaat, E.V.L.; Schulze-Lefert, P. Quantitative divergence of the bacterial root microbiota in Arabidopsis thaliana relatives. Proc. Natl. Acad. Sci. USA. 2014, 111, 585–592. [Google Scholar] [CrossRef]
- Schreiter, S.; Ding, G.-C.; Heuer, H.; Neumann, G.; Sandmann, M.; Grosch, R.; Kropf, S.; Smalla, K. Effect of the soil type on the microbiome in the rhizosphere of field-grown lettuce. Front Microbiol. 2014, 5, 144. [Google Scholar] [CrossRef] [PubMed]
- Hawes, M.C.; Gunawardena, U.; Miyasaka, S.; Zhao, X. The role of root border cells in plant defense. Trends Plant Sci. 2000, 5, 128–133. [Google Scholar] [CrossRef]
- Driouich, A.; Cannesan, M.A.; Dardelle, F.; Durand, C.; Plancot, B.; Bernard, S.; Follet-Gueye, M.L.; Vicré-Gibouin, M. Unity is strength: The power of border cells and border-like cells in relation with plant defense. In Secretions and exudates in biological systems, Signaling and Communication in Plants, 12; Vivanco, J.M., Baluška, F., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 2012; pp. 91–107. [Google Scholar] [CrossRef]
- Liu, Q.; Li, K.; Guo, X.; Ma, L.; Guo, Y.; Liu, Z. Developmental characteristics of grapevine seedlings root border cells and their response to ρ-hydroxybenzoic acid. Plant Soil 2019. [Google Scholar] [CrossRef]
- Knox, O.G.G.; Gupta, V.V.S.R.; Nehl, D.B.; Stiller, W.N. Constitutive expression of cry proteins in roots and border cells of transgenic cotton. Euphytica 2007, 154, 83–90. [Google Scholar] [CrossRef]
- Wuyts, N.; Maung, Z.T.Z.; Swennen, R.; Waele, D.D. Banana rhizodeposition: Characterization of root border cell production and effects on chemotaxis and motility of the parasitic nematode Radopholus similis. Plant Soil 2006, 283, 217–228. [Google Scholar] [CrossRef]
- Kosuta, S.; Chabaud, M.; Lougnon, G.; Gough, C.; Dénarié, J.; Barker, D.G.; Bécard, G. A diffusible factor from arbuscular mycorrhizal fungi induces symbiosis-specific MtENOD11 expression in roots of Medicago truncatula. Plant Physiol. 2003, 131, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Lebeis, S.L.; Paredes, S.H.; Lundberg, D.S.; Breakfield, N.; Gehring, J.; McDonald, M.; Malfatti, S.; Del Rio, T.G.; Jones, C.D.; Tringe, S.G.; et al. Salicylic acid modulates colonization of the root microbiome by specific bacterial taxa. Science 2015, 349, 860–864. [Google Scholar] [CrossRef] [Green Version]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Zamioudis, C.; Pieterse, C.M.J. Modulation of host immunity by beneficial microbes. Mol. Plant. Microbe In. 2012, 25, 139–150. [Google Scholar] [CrossRef]
- Bakker, M.G.; Schlatter, D.C.; Otto-Hanson, L.; Kinkel, L.L. Diffuse symbioses: Roles of plant–plant, plant–microbe and microbe–microbe interactions in structuring the soil microbiome. Mol. Ecol. 2014, 23, 1571–1583. [Google Scholar] [CrossRef]
- Sirrenberg, A.; Göbel, C.; Grond, S.; Czempinski, N.; Ratzinger, A.; Karlovsky, P.; Santos, P.; Feussner, I.; Pawlowski, K. Piriformospora indica affects plant growth by auxin production. Physiol. Plantarum 2007, 131, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Atanasova, L.; Le Crom, S.; Gruber, S.; Coulpier, F.; Seidl-Seiboth, V.; Kubicek, C.P.; Druzhinina, I.S. Comparative transcriptomics reveals different strategies of Trichoderma mycoparasitism. BMC Genomics 2013, 14, 121. [Google Scholar] [CrossRef] [PubMed]
- Čarná, M.; Repka, V.; Skůpa, P.; Šturdík, E. Auxins in defense strategies. Biologia 2014, 69, 1255–1263. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Gan, Y.; Xu, B. Application of plant-growth-promoting fungi Trichoderma longibrachiatum T6 enhances tolerance of wheat to salt stress through improvement of antioxidative defense system and gene expression. Front. Plant Sci. 2016, 7, 1–11. [Google Scholar] [CrossRef]
- Fu, J.; Shiping Wang, S. Insights into auxin signaling in plant–pathogen interactions. Front. Plant Sci. 2011, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, Y. Effects of phosphate solubilization and phytohormone production of Trichoderma asperellum Q1 on promoting cucumber growth under salt stress. J. Integr. Agr. 2015, 14, 1588–1597. [Google Scholar] [CrossRef]
- Nieto-Jacobo, M.F.; Steyaert, J.M.; Salazar-Badillo, F.B.; Nguyen, D.V.; Rostás, D.; Braithwaite, M.; De Souza, J.T.; Jimenez-Bremont, J.F.; Ohkura, M.; Stewart, A.; et al. Environmental growth conditions of Trichoderma spp. affects indole acetic acid derivatives, volatile organic compounds, and plant growth promotion. Front. Plant Sci. 2017, 8, 1–18. [Google Scholar] [CrossRef]
- Geoghegan, I.; Steinberg, G.; Gurr, S. The Role of the Fungal Cell Wall in the Infection of Plants. Trends Microbiol. 2017, 25. [Google Scholar] [CrossRef]
- Fujikawa, T. Surface α-1,3-glucan facilitates fungal stealth infection by interfering with innate immunity in plants. PLoS Pathog. 2012, 8, 16. [Google Scholar] [CrossRef]
- Oliveira-Garcia, E.; Deising, H.B. Infection structure specific expression of β-1,3-glucan synthase is essential for pathogenicity of Colletotrichum graminicola and evasion of β-glucan-triggered immunity in maize. Plant Cell 2013, 25, 2356–2378. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Garcia, E.; Deising, H.B. Attenuation of PAMPtriggered immunity in maize requires down-regulation of the key β-1,6-glucan synthesis genes KRE5 and KRE6 in biotrophic hyphae of Colletotrichum graminicola. Plant J. 2016, 87, 355–375. [Google Scholar] [CrossRef] [PubMed]
- Rovenich, H.; Zuccaro, A.; Thomma, B.P.H.J. Convergent evolution of filamentous microbes towards evasion of glycan-triggered immunity. New Phytol. 2016, 212, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Klarzynski, O.; Plesse, B.; Joubert, J.-M.; Yvin, J.-C.; Kopp, M.; Kloareg, B.; Fritig, B. Linear β-1,3 glucans are elicitors of defense responses in tobacco. Plant Physiol. 2000, 124, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Shetty, N.P.; Jensen, J.D.; Knudsen, A.; Finnie, C.; Geshi, N.; Blennow, A.; Collinge, D.B.; Jørgensen, H.J.L. Effects of β-1,3-glucan from Septoria tritici on structural defence responses in wheat. J. Exp. Bot. 2009, 60, 4287–4300. [Google Scholar] [CrossRef]
- Fesel, P.H.; Zuccaro, A. βeta-glucan: Crucial component of the fungal cell wall and elusive MAMP in plants. Fungal. Genet. Biol. 2016, 90, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Sharpee, W.C.; Dean, R.A. Form and function of fungal and oomycete effectors. Fungal Biol. Rev. 2016, 30, 62–73. [Google Scholar] [CrossRef] [Green Version]
- Marik, T.; Chetna, T.; Dóra, B.; Urbán, P.; Szepesi, Á.; Bakacsy, L.; Endre, G.; Rakk, D.; Szekeres, A.; Andersson, M.A. Structural diversity and bioactivities of peptaibol compounds from Longibrachiatum clade of the filamentous fungal genus Trichoderma. Front. Microbiol. 2019, 10, 1434. [Google Scholar] [CrossRef]
- Sharon, A.; Fuchs, Y.; Anderson, J.D. The elicitation of ethylene biosynthesis by a Trichoderma xylanase is not related to the cell wall degradation activity of the enzyme. Plant Physiol. 1993, 102, 1325–1329. [Google Scholar] [CrossRef]
- Lutz, M.P.; Feichtinger, G.; Ve Défago, G.; Duffy, B. Mycotoxigenic fusarium and deoxynivalenol production repress chitinase gene expression in the biocontrol agent Trichoderma atroviride P1. Appl. Environ. Microbiol. 2003, 69, 3077–3084. [Google Scholar] [CrossRef]
- Röhrich, C.R.; Jaklitsch, W.M.; Voglmayr, H.; Iversen, A.; Vilcinskas, A.; Nielsen, K.F.; Thrane, U.; Von Döhren, H.; Brückner, H.; Degenkolb, T. Front line defenders of the ecological niche! Screening the structural diversity of peptaibiotics from saprotrophic and fungicolous Trichoderma/Hypocrea species. Fungal Divers. 2016, 69, 117–146. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Shahid, M.; Srivastava, M.; Pandey, S.; Sharma, A.; Kumar, V. Optimal physical parameters for growth of Trichoderma species at varying pH, temperature and agitation. Virol. Mycol. 2014, 3, 127. [Google Scholar] [CrossRef]
- Karaoglu, S.A.; Bozdeveci, A.; Pehlivan, N. Characterization of local Trichoderma spp. as potential bio-control agents, screening of in vitro antagonistic activities and fungicide tolerance. J. Biol. Chem. 2018, 46, 247–261. [Google Scholar] [CrossRef]
- Almeida, F.B.; Cerqueira, F.M.; Silva Rdo, N.; Ulhoa, C.J.; Lima, A.L. Mycoparasitism studies of Trichoderma harzianum strains against Rhizoctonia solani: Evaluation of coiling and hydrolytic enzyme production. Biotechnol. Lett. 2007, 29, 1189–1193. [Google Scholar] [CrossRef]
- Borges, M.J.; Azevedo, M.O.; Bonatelli, J.R.; Felipe, M.S.S.; Astolfi-Filho, S. A practical method for the preparation of total DNA from filamentous fungi. Fungal Gen. Newsl. 1990, 37. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [Green Version]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.V.; Singh, N.; Behl, H.M.; Srivastava, S. Influence of plant growth promoting bacteria and its mutant on heavy metal toxicity in Brassica juncea grown in fly ash amended soil. Chemosphere 2008, 72, 678–683. [Google Scholar] [CrossRef]
- Mańka, K. Zbiorowiska grzybów jako kryterium oceny wpływu środowiska na choroby roślin. Zesz. Probl. Post. Nauk. Rol. 1974, 160, 9–23. [Google Scholar]
- Somogyi, M. A new reagent for the determination of sugar. J. Biol. Chem. 1945, 160, 61–68. [Google Scholar]
- Nelson, N. A photometric adaptation of Somogyi method for the determination of glucose. J. Biol. Chem. 1944, 153, 375–380. [Google Scholar]
- Hope, C.F.A.; Burns, R.G. Activity, origins and location of cellulases in a silt loam soil. Biol. Fert. Soils 1987, 5, 164–170. [Google Scholar] [CrossRef]
- Rodriguez-Kabana, R.; Godoy, G.; Morgan-Jones, G.; Shelby, R.A. The determination of soil chitinase activity: Conditions for assay and ecological studies. Plant Soil 1983, 75, 95–106. [Google Scholar] [CrossRef]
- Rössner, H. Bestimmung der Chitinase-Aktivitat. In Bodenbiologische Arbeitsmethoden; Schinner, F., Öhlinger, R., Kandeler, E., Eds.; Springer: Berlin, Germany, 1991. [Google Scholar]
- Bradford, M.M. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Glickmann, E.; Dessaux, Y. A critical examination of the specificity of the Salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl. Environ. Microbiol. 1995, 61, 793–796. [Google Scholar] [PubMed]
- Salkowski, E. Ueber das verhalten der skatolcarbonsaüre im organismus. Z. Physiol. Chem. 1885, 9, 23–33. [Google Scholar]
- Pilet, P.E.; Chollet, R. Colorimetric determination of indole-3-acetic acid. C. R. Acad. Sci. Ser. D. 1970, 271, 1675–1678. [Google Scholar]
- Brückner, B.; Blechschmidt, D.; Recknagel, R.D. Optimalization of nutrient medium for biosynthesis of gibberellic acid. J. Basic Microb. 1991, 31, 243–250. [Google Scholar] [CrossRef]
- Brückner, B. Regulation of gibberellin formation by the fungus Gibberella fujikuroi. In Secondary Metabolites: Their Function and Evolution; Chadwick, D.J., Whelan, J., Eds.; Ciba Foundation Symposium; Wiley: Chichester, UK, 1992; Volume 171, pp. 129–143. [Google Scholar]
- Hasan, H.A.H. Gibberellin and auxin production by plant root-fungi and their biosynthesis under salinity-calcium interaction. Acta Microbiol. Immunol. Hung. 2002, 48, 101–106. [Google Scholar] [CrossRef]
- Tien, T.M.; Gaskins, M.H.; Hubbell, D.H. Plant growth substances produced by Azospirillum bransilense and their effect on the growth of pearl millet (Pennisetumn americanum L.). Appl. Environ. Microbiol. 1979, 37, 1016–1024. [Google Scholar]
- García-Limones, C.; Hervás, A.; Navas-Cortés, J.A.; Jiménez-Díaz, R.M.; Tena, M. Induction of an antioxidant enzyme system and other oxidative stress markers associated with compatible and incompatible interactions between chickpea (Cicer arietinum L.) and Fusarium oxysporum f. sp. ciceris. Physiol. Mol. Plant Pathol. 2002, 61, 325–337. [Google Scholar] [CrossRef]
- Ke, D.; Saltveit, M.E. Spotting and phenylalanine ammonia-lyase activity in iceberg lettuce. Hort Sci. 1986, 21, 1169–1171. [Google Scholar]
- Rösler, J.; Krekel, F.; Amrhein, N.; Schmid, J. Maize phenylalanine ammonia-lyase has tyrosine ammonia-lyase activity. Plant Physiol. 1997, 113, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, O.P.; Van Huystee, R.B. An interrelationship among peroxidase, IAA oxidase, and polyphenol oxidase from peanutcells. Can. J. Bot. 1977, 55, 2630–2635. [Google Scholar] [CrossRef]
- Beers, R.F.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventόs, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Method. Enzymol. 1999, 299, 152–178. [Google Scholar]



















© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaroszuk-Ściseł, J.; Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Majewska, M.; Hanaka, A.; Tyśkiewicz, K.; Pawlik, A.; Janusz, G. Phytohormones (Auxin, Gibberellin) and ACC Deaminase In Vitro Synthesized by the Mycoparasitic Trichoderma DEMTkZ3A0 Strain and Changes in the Level of Auxin and Plant Resistance Markers in Wheat Seedlings Inoculated with this Strain Conidia. Int. J. Mol. Sci. 2019, 20, 4923. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20194923
Jaroszuk-Ściseł J, Tyśkiewicz R, Nowak A, Ozimek E, Majewska M, Hanaka A, Tyśkiewicz K, Pawlik A, Janusz G. Phytohormones (Auxin, Gibberellin) and ACC Deaminase In Vitro Synthesized by the Mycoparasitic Trichoderma DEMTkZ3A0 Strain and Changes in the Level of Auxin and Plant Resistance Markers in Wheat Seedlings Inoculated with this Strain Conidia. International Journal of Molecular Sciences. 2019; 20(19):4923. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20194923
Chicago/Turabian StyleJaroszuk-Ściseł, Jolanta, Renata Tyśkiewicz, Artur Nowak, Ewa Ozimek, Małgorzata Majewska, Agnieszka Hanaka, Katarzyna Tyśkiewicz, Anna Pawlik, and Grzegorz Janusz. 2019. "Phytohormones (Auxin, Gibberellin) and ACC Deaminase In Vitro Synthesized by the Mycoparasitic Trichoderma DEMTkZ3A0 Strain and Changes in the Level of Auxin and Plant Resistance Markers in Wheat Seedlings Inoculated with this Strain Conidia" International Journal of Molecular Sciences 20, no. 19: 4923. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20194923