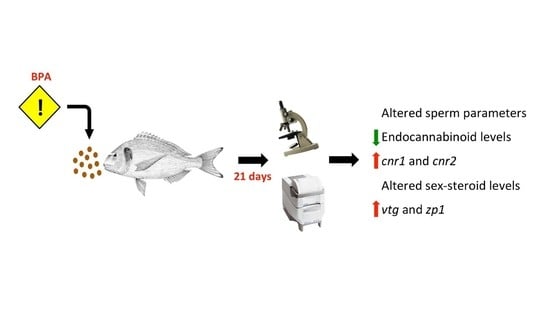
Effects of Dietary Bisphenol A on the Reproductive Function of Gilthead Sea Bream (Sparus aurata) Testes
Abstract
:1. Introduction
2. Results
2.1. BPA Increased GSI and Altered Sperm Quality
2.2. Gonadal Morphology
2.3. Altered Endocannabinoid and Endocannabinoid-Like Mediator Levels in the Testis
2.4. Sex Steroid Levels
2.5. Modifications at The Transcriptomic Level in The Male Sea Bream Liver and Testis
3. Discussion
4. Materials and Methods
4.1. Fish Maintenance, Food Preparation and Animal Treatment
4.2. Sperm Quality Evaluation
4.3. Testes Histology
4.4. Enzyme-Linked ImmunoSorbent Assay (ELISA)
4.5. RNA Extraction, cDNA Synthesis and Real-Time PCR
4.6. Concentration of Endogenous Cannabinoids (Anandamide (AEA), 2-Arachidonoylglycerol (2-AG)), Endocannabinoid-Like Mediators (Palmitoylethanolamide (PEA), Oleoylethanolamide (OEA)) and Fatty Acid Amide Hydrolase (FAAH) Enzymatic Activity in the Testis
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Crain, D.A.; Eriksen, M.; Iguchi, T.; Jobling, S.; Laufer, H.; LeBlanc, G.A.; Guillette, L.J. An ecological assessment of bisphenol-A: Evidence from comparative biology. Reprod. Toxicol. 2007, 24, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Williams & Marshall Strategy. The Global Bisphenol A Market. 2019. Available online: https://www.researchandmarkets.com/research/hl86rz/global_bisphenol?w=5 (accessed on 12 March 2019).
- Corrales, J.; Kristofco, L.A.; Steele, W.B.; Yates, B.S.; Breed, C.S.; Williams, E.S.; Brooks, B.W. Global Assessment of Bisphenol A in the Environment. Dose-Response 2015, 13, 155932581559830. [Google Scholar] [CrossRef] [PubMed]
- Fromme, H.; Küchler, T.; Otto, T.; Pilz, K.; Müller, J.; Wenzel, A. Occurrence of phthalates and bisphenol A and F in the environment. Water Res. 2002, 36, 1429–1438. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Maffini, M.V.; Sonnenschein, C.; Rubin, B.S.; Soto, A.M. Bisphenol-A and the great divide: A review of controversies in the field of endocrine disruption. Endocr. Rev. 2009, 30, 75–95. [Google Scholar] [CrossRef] [PubMed]
- Staples, C.A.; Dome, P.B.; Klecka, G.M.; Oblock, S.T.; Harris, L.R. A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere 1998, 36, 2149–2173. [Google Scholar] [CrossRef]
- Lindholst, C.; Pedersen, S.N.; Bjerregaard, P. Uptake, metabolism and excretion of bisphenol A in the rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 2001, 55, 75–84. [Google Scholar] [CrossRef]
- Mita, L.; Bianco, M.; Viggiano, E.; Zollo, F.; Bencivenga, U.; Sica, V.; Monaco, G.; Portaccio, M.; Diano, N.; Colonna, A.; et al. Bisphenol A content in fish caught in two different sites of the Tyrrhenian Sea (Italy). Chemosphere 2011, 82, 405–410. [Google Scholar] [CrossRef]
- Lee, C.-C.; Jiang, L.-Y.; Kuo, Y.-L.; Chen, C.-Y.; Hsieh, C.-Y.; Hung, C.-F.; Tien, C.-J. Characteristics of nonylphenol and bisphenol A accumulation by fish and implications for ecological and human health. Sci. Total Environ. 2015, 502, 417–425. [Google Scholar] [CrossRef]
- Mandich, A.; Bottero, S.; Benfenati, E.; Cevasco, A.; Erratico, C.; Maggioni, S.; Massari, A.; Pedemonte, F.; Viganò, L. In vivo exposure of carp to graded concentrations of bisphenol A. Gen. Comp. Endocrinol. 2007, 153, 15–24. [Google Scholar] [CrossRef]
- Hatef, A.; Zare, A.; Alavi, S.M.H.; Habibi, H.R.; Linhart, O. Modulations in androgen and estrogen mediating genes and testicular response in male goldfish exposed to bisphenol A. Environ. Toxicol. Chem. 2012, 31, 2069–2077. [Google Scholar] [CrossRef]
- Hatef, A.; Alavi, S.M.H.; Abdulfatah, A.; Fontaine, P.; Rodina, M.; Linhart, O. Adverse effects of bisphenol A on reproductive physiology in male goldfish at environmentally relevant concentrations. Ecotoxicol. Environ. Saf. 2012, 76, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Lahnsteiner, F.; Berger, B.; Kletzl, M.; Weismann, T. Effect of bisphenol A on maturation and quality of semen and eggs in the brown trout, Salmo trutta f. fario. Aquat. Toxicol. 2005, 75, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Sohoni, P.; Tyler, C.R.; Hurd, K.; Caunter, J.; Hetheridge, M.; Williams, T.; Woods, C.; Evans, M.; Toy, R.; Gargas, M.; et al. Reproductive Effects of Long-Term Exposure to Bisphenol A in the Fathead Minnow (Pimephales promelas). Environ. Sci. Technol. 2001, 35, 2917–2925. [Google Scholar] [CrossRef] [PubMed]
- Bjerregaard, L.B.; Madsen, A.H.; Korsgaard, B.; Bjerregaard, P. Gonad histology and vitellogenin concentrations in brown trout (Salmo trutta) from Danish streams impacted by sewage effluent. Ecotoxicology 2006, 15, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Maradonna, F.; Nozzi, V.; Dalla Valle, L.; Traversi, I.; Gioacchini, G.; Benato, F.; Colletti, E.; Gallo, P.; Di Marco Pisciottano, I.; Mita, D.G.; et al. A developmental hepatotoxicity study of dietary bisphenol A in Sparus aurata juveniles. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2014, 166, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Santangeli, S.; Maradonna, F.; Gioacchini, G.; Cobellis, G.; Piccinetti, C.C.; Dalla Valle, L.; Carnevali, O. BPA-Induced deregulation of epigenetic patterns: Effects on female zebrafish reproduction. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Santangeli, S.; Maradonna, F.; Olivotto, I.; Piccinetti, C.C.; Gioacchini, G.; Carnevali, O. Effects of BPA on female reproductive function: The involvement of epigenetic mechanism. Gen. Comp. Endocrinol. 2017, 245. [Google Scholar] [CrossRef] [PubMed]
- Witorsch, R.J. Endocrine Disruptors: Can Biological Effects and Environmental Risks Be Predicted? Regul. Toxicol. Pharmacol. 2002, 36, 118–130. [Google Scholar] [CrossRef]
- Oehlmann, J.; Schulte-Oehlmann, U.; Kloas, W.; Jagnytsch, O.; Lutz, I.; Kusk, K.O.; Wollenberger, L.; Santos, E.M.; Paull, G.C.; Van Look, K.J.W.; et al. A critical analysis of the biological impacts of plasticizers on wildlife. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2047–2062. [Google Scholar] [CrossRef] [Green Version]
- Gorzalka, B.B.; Dang, S.S. Minireview: Endocannabinoids and Gonadal Hormones: Bidirectional Interactions in Physiology and Behavior. Endocrinology 2012, 153, 1016–1024. [Google Scholar] [CrossRef] [Green Version]
- Forner-Piquer, I.; Santangeli, S.; Maradonna, F.; Rabbito, A.; Piscitelli, F.; Habibi, H.R.; Di, V. Disruption of the gonadal endocannabinoid system in zebra fish exposed to diisononyl phthalate. Environ. Pollut. 2018, 241, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cottone, E.; Pomatto, V.; Bovolin, P. Role of the endocannabinoid system in the central regulation of nonmammalian vertebrate reproduction. Int. J. Endocrinol. 2013, 2013, 941237. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, B.; Soverchia, L.; Mosconi, G.; Franzoni, M.F.; Cottone, E.; Polzonetti-Magni, A.M. Changes of gonadal CB1 cannabinoid receptor mRNA in the gilthead seabream, Sparus aurata, during sex reversal. Gen. Comp. Endocrinol. 2007, 150, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Cottone, E.; Guastalla, A.; Mackie, K.; Franzoni, M.F. Endocannabinoids affect the reproductive functions in teleosts and amphibians. Mol. Cell. Endocrinol. 2008, 286, S41–S45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battista, N.; Meccariello, R.; Cobellis, G.; Fasano, S.; Di Tommaso, M.; Pirazzi, V.; Konje, J.C.; Pierantoni, R.; Maccarrone, M. The role of endocannabinoids in gonadal function and fertility along the evolutionary axis. Mol. Cell. Endocrinol. 2012, 355, 1–14. [Google Scholar] [CrossRef]
- Grimaldi, P.; Di Giacomo, D.; Geremia, R. The endocannabinoid system and spermatogenesis. Front. Endocrinol. (Lausanne). 2013, 4, 192. [Google Scholar] [CrossRef]
- Maccarrone, M.; Bab, I.; Bíró, T.; Cabral, G.A.; Dey, S.K.; Di Marzo, V.; Konje, J.C.; Kunos, G.; Mechoulam, R.; Pacher, P.; et al. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol. Sci. 2015, 36, 277–296. [Google Scholar] [CrossRef] [Green Version]
- Zohar, Y.; Abraham, M.; Gordin, H. The gonadal cycle of the captivity-reared hermaphroditic teleost Sparus aurata (L.) during the first two years of life. Ann. Biol. Anim. Bioch. Biophys. 1978, 18, 877–882. [Google Scholar] [CrossRef]
- Welshons, W.V.; Nagel, S.C.; vom Saal, F.S. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology 2006, 147, s56–s69. [Google Scholar] [CrossRef]
- Organisation for Economic Co-Operation and Development. Number 60. Report of the initial work towards the validation of the 21-day fish screening assay for the detection of endocrine active substances (Phase 1A). 2006. Available online: http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2006)27&doclanguage=en (accessed on 5 June 2019).
- Zhang, Y.; Yuan, C.; Hu, G.; Li, M.; Zheng, Y.; Gao, J.; Yang, Y.; Zhou, Y.; Wang, Z. Characterization of four nr5a genes and gene expression profiling for testicular steroidogenesis-related genes and their regulatory factors in response to bisphenol A in rare minnow Gobiocypris rarus. Gen. Comp. Endocrinol. 2013, 194, 31–44. [Google Scholar] [CrossRef]
- Kang, I.J.; Yokota, H.; Oshima, Y.; Tsuruda, Y.; Oe, T.; Imada, N.; Tadokoro, H.; Honjo, T. Effects of bisphenol a on the reproduction of Japanese medaka (Oryzias latipes). Environ. Toxicol. Chem. 2002, 21, 2394–2400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guan, Y.; Zhang, T.; Yuan, C.; Liu, Y.; Wang, Z. Adult exposure to bisphenol A in rare minnow Gobiocypris rarus reduces sperm quality with disruption of testicular aquaporins. Chemosphere 2018, 193, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Bjerregaard, P.; Andersen, S.B.; Pedersen, K.L.; Pedersen, S.N.; Korsgaard, B. Orally administered bisphenol a in rainbow trout (Oncorhynchus mykiss): EStrogenicity, metabolism, and retention. Environ. Toxicol. Chem. 2007, 26, 1910. [Google Scholar] [CrossRef] [PubMed]
- Jurgella, G.F.; Marwah, A.; Malison, J.A.; Peterson, R.; Barry, T.P. Effects of xenobiotics and steroids on renal and hepatic estrogen metabolism in lake trout. Gen. Comp. Endocrinol. 2006, 148, 273–281. [Google Scholar] [CrossRef]
- Hiroi, H.; Tsutsumi, O.; Momoeda, M.; Takai, Y.; Osuga, Y.; Taketani, Y. Differential interactions of bisphenol A and 17 beta-estradiol with estrogen receptor alpha (ERalpha) and ERbeta. Endocr. J. 1999, 46, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, C.; Gao, J.; Liu, Y.; Wang, Z. Testicular transcript responses in rare minnow Gobiocypris rarus following different concentrations bisphenol A exposure. Chemosphere 2016, 156, 357–366. [Google Scholar] [CrossRef]
- Schulz, R.W.; de França, L.R.; Lareyre, J.-J.; LeGac, F.; Chiarini-Garcia, H.; Nobrega, R.H.; Miura, T. Spermatogenesis in fish. Gen. Comp. Endocrinol. 2010, 165, 390–411. [Google Scholar] [CrossRef] [PubMed]
- Cabas, I.; Chaves-Pozo, E.; García-Alcázar, A.; Meseguer, J.; Mulero, V.; García-Ayala, A. The effect of 17α-ethynylestradiol on steroidogenesis and gonadal cytokine gene expression is related to the reproductive stage in marine hermaphrodite fish. Mar. Drugs 2013, 11, 4973–4992. [Google Scholar] [CrossRef]
- Mendez-Sanchez, N.; Zamora-Valdes, D.; Pichardo-Bahena, R.; Barredo-Prieto, B.; Ponciano-Rodriguez, G.; Bermejo-Martínez, L.; Chavez-Tapia, N.C.; Baptista-González, H.A.; Uribe, M. Endocannabinoid receptor CB2 in nonalcoholic fatty liver disease. Liver Int. 2007, 27, 215–219. [Google Scholar] [CrossRef]
- Scott, A.P.; Sumpter, J.P.; Stacey, N. The role of the maturation-inducing steroid, 17,20β-dihydroxypregn-4-en-3-one, in male fishes: A review. J. Fish Biol. 2010, 76, 183–224. [Google Scholar] [CrossRef]
- Mihaich, E.; Rhodes, J.; Wolf, J.; van der Hoeven, N.; Dietrich, D.; Hall, A.T.; Caspers, N.; Ortego, L.; Staples, C.; Dimond, S.; et al. Adult fathead minnow, Pimephales promelas, partial life-cycle reproductive and gonadal histopathology study with bisphenol A. Environ. Toxicol. Chem. 2012, 31, 2525–2535. [Google Scholar] [CrossRef] [PubMed]
- Forner-Piquer, I.; Mylonas, C.C.; Fakriadis, I.; Papadaki, M.; Piscitelli, F.; Di Marzo, V.; Calduch-Giner, J.; Pérez-Sánchez, J.; Carnevali, O. Effects of diisononyl phthalate (DiNP) on the endocannabinoid and reproductive systems of male gilthead sea bream (Sparus aurata) during the spawning season. Arch. Toxicol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Hatef, A.; Alavi, S.M.H.; Linhartova, Z.; Rodina, M.; Policar, T.; Linhart, O. In vitro effects of Bisphenol A on sperm motility characteristics in Perca fluviatilis L. (Percidae; Teleostei). J. Appl. Ichthyol. 2010, 26, 696–701. [Google Scholar] [CrossRef]
- Rouxel, C.; Suquet, M.; Cosson, J.; Severe, A.; Quemener, L.; Fauvel, C. Changes in Atlantic cod (Gadus morhua L.) sperm quality during the spawning season. Aquac. Res. 2008, 39, 434–440. [Google Scholar] [CrossRef]
- Schuel, H.; Schuel, R.; Zimmerman, A.M.; Zimmerman, S. Cannabinoids reduce fertility of sea urchin sperm. Biochem. Cell Biol. 1987, 65, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Rapino, C.; Battista, N.; Bari, M.; Maccarrone, M. Endocannabinoids as biomarkers of human reproduction. Hum. Reprod. Update 2014, 20, 501–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bovolin, P.; Cottone, E.; Pomatto, V.; Fasano, S.; Pierantoni, R.; Cobellis, G.; Meccariello, R. Endocannabinoids are involved in male vertebrate reproduction: Regulatory mechanisms at central and gonadal level. Front. Endocrinol. (Lausanne) 2014, 5, 54. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, P.; Pucci, M.; Di Siena, S.; Di Giacomo, D.; Pirazzi, V.; Geremia, R.; Maccarrone, M. The faah gene is the first direct target of estrogen in the testis: Role of histone demethylase LSD1. Cell. Mol. Life Sci. 2012, 69, 4177–4190. [Google Scholar] [CrossRef]
- Rossi, G.; Gasperi, V.; Paro, R.; Barsacchi, D.; Cecconi, S.; Maccarrone, M. Follicle-stimulating hormone activates fatty acid amide hydrolase by protein kinase A and aromatase-dependent pathways in mouse primary sertoli cells. Endocrinology 2007, 148, 1431–1439. [Google Scholar] [CrossRef]
- Di Marzo, V.; Bisogno, T.; Sugiura, T.; Melck, D.; De Petrocellis, L. The novel endogenous cannabinoid 2-arachidonoylglycerol is inactivated by neuronal- and basophil-like cells: Connections with anandamide. Biochem. J. 1998, 331 Pt 1, 15–19. [Google Scholar] [CrossRef]
- Chalmel, F.; Rolland, A.D. Linking transcriptomics and proteomics in spermatogenesis. Reproduction 2015, 150, R149–R157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geremia, R.; Boitani, C.; Conti, M.; Monesi, V. RNA synthesis in spermatocytes and spermatids and preservation of meiotic RNA during spermiogenesis in the mouse. Cell Differ. 1977, 5, 343–355. [Google Scholar] [CrossRef]
- Lewis, S.E.M.; Rapino, C.; Di Tommaso, M.; Pucci, M.; Battista, N.; Paro, R.; Simon, L.; Lutton, D.; Maccarrone, M. Differences in the endocannabinoid system of sperm from fertile and infertile men. PLoS ONE 2012, 7, e47704. [Google Scholar] [CrossRef]
- Amoako, A.A.; Marczylo, T.H.; Marczylo, E.L.; Elson, J.; Willets, J.M.; Taylor, A.H.; Konje, J.C. Anandamide modulates human sperm motility: Implications for men with asthenozoospermia and oligoasthenoteratozoospermia. Hum. Reprod. 2013, 28, 2058–2066. [Google Scholar] [CrossRef] [PubMed]
- Barbonetti, A.; Vassallo, M.R.C.; Fortunato, D.; Francavilla, S.; Maccarrone, M.; Francavilla, F. Energetic metabolism and human sperm motility: Impact of CB₁ receptor activation. Endocrinology 2010, 151, 5882–5892. [Google Scholar] [CrossRef]
- Chioccarelli, T.; Cacciola, G.; Altucci, L.; Lewis, S.E.M.; Simon, L.; Ricci, G.; Ledent, C.; Meccariello, R.; Fasano, S.; Pierantoni, R.; et al. Cannabinoid receptor 1 influences chromatin remodeling in mouse spermatids by affecting content of transition protein 2 mRNA and histone displacement. Endocrinology 2010, 151, 5017–5029. [Google Scholar] [CrossRef] [PubMed]
- Bari, M.; Battista, N.; Pirazzi, V.; Maccarrone, M. The manifold actions of endocannabinoids on female and male reproductive events. Front. Biosci. (Landmark Ed.) 2011, 16, 498–516. [Google Scholar] [CrossRef] [Green Version]
- Forner-Piquer, I.; Mylonas, C.C.; Calduch-Giner, J.; Maradonna, F.; Gioacchini, G.; Allarà, M.; Piscitelli, F.; Di Marzo, V.; Pérez-Sánchez, J.; Carnevali, O. Endocrine disruptors in the diet of male Sparus aurata: Modulation of the endocannabinoid system at the hepatic and central level by Di-isononyl phthalate and Bisphenol A. Environ. Int. 2018, 119, 54–65. [Google Scholar] [CrossRef]
- Organisation for Economic Co-Operation and Development. Number 78. Final Report of the Validation of the 21-day Fish Screening Assay for the Detection of Endocrine Active Substances. Phase 2: Testing Negative Substances. 2007. Available online: http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2006)27&doclanguage=en (accessed on 5 June 2019).
- Organisation for Economic Co-Operation and Development. Number 109. Literature Review on the 21-day Fish Assay and the Fish Short-term Reproduction Assay. 2009. Available online: http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2009)14&doclanguage=en (accessed on 5 June 2019).
- Mylonas, C.C.; Cardinaletti, G.; Sigelaki, I.; Polzonetti-Magni, A. Comparative efficacy of clove oil and 2-phenoxyethanol as anesthetics in the aquaculture of European sea bass (Dicentrarchus labrax) and gilthead sea bream (Sparus aurata) at different temperatures. Aquaculture 2005, 246, 467–481. [Google Scholar] [CrossRef]
- Papadaki, M.; Mazzella, D.; Santinelli, V.; Fakriadis, I.; Sigelaki, I.; Mylonas, C.C. Hermaphroditism and reproductive function of hatchery-produced sharpsnout seabream (Diplodus puntazzo) under attenuated annual thermal cycles. Aquaculture 2018, 482, 231–240. [Google Scholar] [CrossRef]
- Bennett, H.S.; Wyrick, A.D.; Lee, S.W.; McNeil, J.H. Science and art in preparing tissues embedded in plastic for light microscopy, with special reference to glycol methacrylate, glass knives and simple stains. Stain Technol. 1976, 51, 71–97. [Google Scholar] [CrossRef] [PubMed]
- Nash, J.P.; Cuisset, B.D.; Bhattacharyya, S.; Suter, H.C.; Le Menn, F.; Kime, D.E. An enzyme linked immunosorbant assay (ELISA) for testosterone, estradiol, and 17,20β-dihydroxy-4-pregenen-3-one using acetylcholinesterase as tracer: Application to measurement of diel patterns in rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 2000, 22, 355–363. [Google Scholar] [CrossRef]
- Rodríguez, L.; Begtashi, I.; Zanuy, S.; Carrillo, M. Development and validation of an enzyme immunoassay for testosterone: Effects of photoperiod on plasma testosterone levels and gonadal development in male sea bass (Dicentrarchus labrax, L.) at puberty. Fish Physiol. Biochem. 2000, 23, 141–150. [Google Scholar] [CrossRef]







| Gene | POST-CTRL | BPA LOW | BPA HIGH |
|---|---|---|---|
| cnr1 | 0.38 ± 0.07 | 0.64 ± 0.10 * | 0.60 ± 0.06 |
| cnr2 | 0.35 ± 0.04 | 0.24 ± 0.04 | 0.63 ± 0.08 ** |
| ppar α | 3.33 ± 0.34 | 2.43 ± 0.22 | 2.49 ± 0.24 |
| ppar β | 6.41 ± 0.63 | 6.82 ± 0.80 | 6.86 ± 0.72 |
| ppar γ | 12.68 ± 1.69 | 16.14 ± 2.13 | 12.63 ± 1.52 |
| trpv1 | 0.24 ± 0.03 | 0.37 ± 0.03 * | 0.30 ± 0.03 |
| faah | 4.87 ± 0.49 | 6.81 ± 0.67 | 4.81 ± 0.62 |
| nape-pld | 22.71 ± 1.55 | 25.50 ± 2.50 | 19.29 ± 1.94 |
| abdh4 | 3.69 ± 0.35 | 2.52 ± 0.35* | 3.00 ± 0.19 |
| cyt-pla2 | 1.00 ± 0.12 | 0.94 ± 0.12 | 1.22 ± 0.15 |
| cox2 | 1.43 ± 0.18 | 1.13 ± 0.12 | 0.98 ± 0.12 |
| daglα | 4.39 ± 0.46 | 5.96 ± 0.64 | 4,17 ± 0.49 |
| abdh 6a | 4.42 ± 0.34 | 3.56 ± 0.35 | 4.00 ± 0.36 |
| abdh 12b | 2.56 ± 0.29 | 2.65 ± 0.33 | 2.54 ± 0.34 |
| lepr | 15.88 ± 2.80 | 51.49 ± 5.16 **** | 24.75 ± 4.78 |
| lepa | 0.30 ± 0.03 | 0.25 ± 0.03 | 0.37 ± 0.04 |
| era | 3.04 ± 0.30 | 4.23 ± 0.34 * | 4.07 ± 0.33 |
| erb | 11.89 ± 0.67 | 11.68 ± 1.13 | 15.33 ± 1.02 * |
| pr | 4.36 ± 0.60 | 5.67 ± 0.66 | 7.57 ± 0.65 ** |
| ar | 8.59 ± 0.78 | 7.28 ± 0.89 | 7.72 ± 0.67 |
| lhr | 0.56 ± 0.06 | 0.50 ± 0.09 | 0.61 ± 0.04 |
| fshr | 0.46 ± 0.10 | 0.63 ± 0.07 | 0.56 ± 0.07 |
| gnrhr | 5.36 ± 0.81 | 5.11 ± 0.49 | 8.44 ± 0.96 * |
| 17b-hsd | 5.20 ± 0.50 | 4.75 ± 0.53 | 4.85 ± 0.45 |
| 3b-hsd | 5.91 ± 0.59 | 6.80 ± 0.62 | 5.63 ± 0.76 |
| Gene | POST-CTRL | BPA LOW | BPA HIGH |
|---|---|---|---|
| vtga | 0.76 ± 0.43 | 1.71 ± 0.43 ** | 0.96 ± 0.33 |
| zp1 | 1.00 ± 0.35 | 1.91 ± 0.48 * | 1.00 ± 0.26 |
| zp3 | 116.32 ± 39.82 | 58.51 ± 37.54 | 73.11 ± 35.97 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forner-Piquer, I.; Fakriadis, I.; Mylonas, C.C.; Piscitelli, F.; Di Marzo, V.; Maradonna, F.; Calduch-Giner, J.; Pérez-Sánchez, J.; Carnevali, O. Effects of Dietary Bisphenol A on the Reproductive Function of Gilthead Sea Bream (Sparus aurata) Testes. Int. J. Mol. Sci. 2019, 20, 5003. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20205003
Forner-Piquer I, Fakriadis I, Mylonas CC, Piscitelli F, Di Marzo V, Maradonna F, Calduch-Giner J, Pérez-Sánchez J, Carnevali O. Effects of Dietary Bisphenol A on the Reproductive Function of Gilthead Sea Bream (Sparus aurata) Testes. International Journal of Molecular Sciences. 2019; 20(20):5003. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20205003
Chicago/Turabian StyleForner-Piquer, Isabel, Ioannis Fakriadis, Constantinos C Mylonas, Fabiana Piscitelli, Vincenzo Di Marzo, Francesca Maradonna, Josep Calduch-Giner, Jaume Pérez-Sánchez, and Oliana Carnevali. 2019. "Effects of Dietary Bisphenol A on the Reproductive Function of Gilthead Sea Bream (Sparus aurata) Testes" International Journal of Molecular Sciences 20, no. 20: 5003. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20205003