Antioxidant, Anti-Inflammatory, and Metabolic Properties of Tocopherols and Tocotrienols: Clinical Implications for Vitamin E Supplementation in Diabetic Kidney Disease
Abstract
:1. Introduction
2. Diabetes, Oxidative Stress, and Inflammation
2.1. Diabetic Kidney Disease
2.2. Oxidative Stress
2.3. Pro-Inflammatory Status
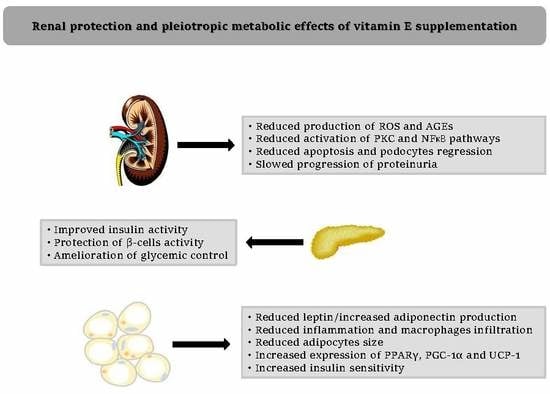
3. Diabetic Nephropathy and Vitamin E
3.1. Vitamin E and Diabetic Nephropathy: Clinical Evidence
3.2. Vitamin E and Diabetic Nephropathy: Antioxidants and Anti-Inflammatory Mechanisms
3.3. Vitamin E and Diabetic Nephropathy: Metabolic Mechanisms
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ASCVD | Atherosclerotic cardiovascular disease |
| LDL | Low-density lipoprotein |
| IU | International Unit |
| DKD | Diabetic kidney disease |
| MICRO-HOPE | Microalbuminuria, Cardiovascular and Renal Outcomes–Heart Outcomes Prevention Evaluation |
| ESRD | End-stage renal disease |
| ROS | Reactive oxygen species |
| AGEs | Advanced glycation end products |
| PKC | Protein kinase C |
| NFκB | Nuclear factor κB |
| e-NOS | Endothelial-nitric oxide synthase |
| vWF | Von Willebrand factor |
| PAI-1 | Plasminogen activator inhibitor-1 |
| RAGEs | Receptors for advanced glycation end products |
| IL-1 | Interleukin-1 |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor necrosis factor-α |
| VCAM-1 | Vascular adhesion molecular-1 |
| ICAM-1 | Intercellular adhesion molecule-1 |
| MCP-1 | Monocyte chemoattractant protein-1 |
| RCTs | Randomized controlled trials |
| HOMA | Homeostasis Model Assessment |
| FMD | Flow-mediated dilation |
| SMD | Standardized mean difference |
| Hp | Haptoglobin genotype |
| MMP | Matrix metalloproteinase |
References
- GISSI-Prevenzione Investigators. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: Results of the GISSI-Prevenzione trial. Lancet 1999, 354, 447–455. [Google Scholar] [CrossRef]
- Lonn, E.; Yusuf, S.; Dzavik, V.; Doris, C.; Yi, Q.; Smith, S.; Moore-Cox, A.; Bosch, J.; Riley, W.; Teo, K. SECURE Investigators. Effects of ramipril and vitamin E on atherosclerosis: The study to evaluate carotid ultrasound changes in patients treated with ramipril and vitamin E (SECURE). Circulation 2001, 103, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Sleight, P.; Pogue, J.; Bosch, J.; Davies, R.; Dagenais, G. Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N. Engl. J. Med. 2000, 342, 145–153. [Google Scholar] [PubMed]
- Mathur, P.; Ding, Z.; Saldeen, T.; Mehta, J.L. Tocopherols in the Prevention and Treatment of Atherosclerosis and Related Cardiovascular Disease. Clin. Cardiol. 2015, 38, 570–576. [Google Scholar] [CrossRef]
- Dotan, Y.; Pinchuk, I.; Lichtenberg, D.; Leshno, M. Decision analysis supports the paradigm that indiscriminate supplementation of vitamin E does more harm than good. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1304–1309. [Google Scholar] [CrossRef]
- Beckman, J.A.; Creager, M.A. Vascular Complications of Diabetes. Circ. Res. 2016, 118, 1771–1785. [Google Scholar] [CrossRef] [Green Version]
- Tervaert, T.W.; Mooyaart, A.L.; Amann, K.; Cohen, A.H.; Cook, H.T.; Drachenberg, C.B.; Ferrario, F.; Fogo, A.B.; Haas, M.; de Heer, E. Pathologic classifcation of diabetic nephropathy. J. Am. Soc. Nephrol. 2014, 21, 556–563. [Google Scholar] [CrossRef]
- Giorda, C.B.; Carna, P.; Salomone, M.; Picariello, R.; Costa, G.; Tartaglino, B.; Gnavi, R. Ten-year comparative analysis of incidence, prognosis, and associated factors for dialysis and renal transplantation in type 1 an d type 2 diabetes versus non-diabetes. Acta Diabetol. 2018, 55, 733–740. [Google Scholar] [CrossRef]
- Soleymani, H.; Saboury, A.A.; Moosavi-Movahedi, A.A.; Rahmani, F.; Maleki, J.; Yousefinejad, S.; Maghami, P. Vitamin E induces regular structure and stability of human insulin, more intense than vitamin D3. Int. J. Biol. Macromol. 2016, 93, 868–878. [Google Scholar] [CrossRef] [Green Version]
- Chia, L.L.; Jantan, I.; Chua, K.H. Tocotrienols Stimulate Insulin Secretion of Rat Pancreatic Isolated Islets in a Dynamic Culture. Curr. Pharm. Biotechnol. 2017, 18, 560–568. [Google Scholar] [CrossRef]
- Korshunov, S.S.; Skulachev, V.P.; Starkov, A.A. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997, 416, 15–18. [Google Scholar] [CrossRef] [Green Version]
- Tuttle, K.R.; Bakris, G.L.; Toto, R.D.; McGill, J.B.; Hu, K.; Anderson, P.W. The effect of ruboxistaurin on nephropathy in type 2 diabetes. Diabetes Care 2005, 28, 2686–2690. [Google Scholar] [CrossRef] [PubMed]
- Christ, M.; Bauersachs, J.; Liebetrau, C.; Heck, M.; Günther, A.; Wehling, M. Glucose increases endothelial-dependent superoxide formation in coronary arteries by NAD(P)H oxidase activation: Attenuation by the 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor atorvastatin. Diabetes 2002, 51, 2648–2652. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bharath, L.P.; Qian, Y.; Ruan, T.; Anandh Babu, P.V.; Bruno, R.S.; Symons, J.D.; Jalili, T. γ-Carboxyethyl hydroxychroman, a metabolite of γ-tocopherol, preserves nitric oxide bioavailability in endothelial cells challenged with high glucose. Exp. Biol. Med. 2016, 241, 2056–2062. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ananthakrishnan, R.; Qu, W.; Lu, Y.; Reiniger, N.; Zeng, S.; Ma, W.; Rosario, R.; Yan, S.F.; Ramasamy, R.; et al. RAGE mediates podocyte injury in adriamycin-induced glomerulosclerosis. J. Am. Soc. Nephrol. 2008, 19, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Kajitani, N.; Shikata, K.; Nakamura, A.; Nakatou, T.; Hiramatsu, M.; Makino, H. Microinflammation is a common risk factor for progression of nephropathy and atherosclerosis in Japanese patients with type 2 diabetes. Diabetes Res. Clin. Pract. 2010, 88, 171–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiarelli, F.; Cipollone, F.; Mohn, A.; Marini, M.; Iezzi, A.; Fazia, M.; Tumini, S.; De Cesare, D.; Pomilio, M.; Pierdomenico, S.D.; et al. Circulating monocyte chemoattractant protein-1 and early development of nephropathy in type 1 diabetes. Diabetes Care 2002, 25, 1829–1834. [Google Scholar] [CrossRef]
- Steinberg, D.; Witztum, J.L. Oxidized low-density lipoprotein and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2311–2316. [Google Scholar] [CrossRef]
- El-Aal, A.A.; El-Ghffar, E.A.A.; Ghali, A.A.; Zughbur, M.R.; Sirdah, M.M. The effect of vitamin C and/or E supplementations on type 2 diabetic adult males under metformin treatment: A single-blinded randomized controlled clinical trial. Diabetes Metab. Syndr. 2018, 12, 483–489. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, S.; Tao, A.; Chen, G.; Zhang, M. Influence of vitamin E supplementation on glycaemic control: A meta-analysis of randomised controlled trials. PLoS ONE 2014, 9, e95008. [Google Scholar] [CrossRef]
- Maktabi, M.; Jamilian, M.; Amirani, E.; Chamani, M.; Asemi, Z. The effects of magnesium and vitamin E co-supplementation on parameters of glucose homeostasis and lipid profiles in patients with gestational diabetes. Lipids Health Dis. 2018, 17, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mune, M.; Uto-Kondo, H.; Iteya, I.; Fujii, Y.; Ikeda, S.; Ikewaki, K. Vitamin E supplementation improves high-density lipoprotein and endothelial functions in end-stage kidney disease patients undergoing hemodialysis. Clin. Nephrol. 2018, 90, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Ashor, A.W.; Siervo, M.; Lara, J.; Oggioni, C.; Afshar, S.; Mathers, J.C. Effect of vitamin C and vitamin E supplementation on endothelial function: A systematic review and meta-analysis of randomised controlled trials. Br. J. Nutr. 2015, 113, 1182–1194. [Google Scholar] [CrossRef] [PubMed]
- Bolignano, D.; Cernaro, V.; Gembillo, G.; Baggetta, R.; Buemi, M.; D’Arrigo, G. Antioxidant agents for delaying diabetic kidney disease progression: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0178699. [Google Scholar] [CrossRef] [PubMed]
- Gaede, P.; Poulsen, H.E.; Parving, H.H.; Pedersen, O. Double-blind, randomised study of the effect of combined treatment with vitamin C and E on albuminuria in Type 2 diabetic patients. Diabet. Med. 2001, 18, 756–760. [Google Scholar] [CrossRef]
- Tan, S.M.Q.; Chiew, Y.; Ahmad, B.; Kadir, K.A. Tocotrienol-Rich Vitamin E from Palm Oil (Tocovid) and Its Effects in Diabetes and Diabetic Nephropathy: A Pilot Phase II Clinical Trial. Nutrients 2018, 10, 1315. [Google Scholar] [CrossRef]
- Boaz, M.; Smetana, S.; Weinstein, T.; Matas, Z.; Gafter, U.; Iaina, A.; Knecht, A.; Weissgarten, Y.; Brunner, D.; Fainaru, M.; et al. Secondary prevention with antioxidants of cardiovascular disease in endstage renal disease (SPACE): Randomised placebo-controlled trial. Lancet 2000, 356, 1213–1218. [Google Scholar] [CrossRef]
- Aghadavod, E.; Soleimani, A.; Hamidi, G.; Keneshlou, F.; Heidari, A.; Asemi, Z. Effects of High-dose Vitamin E Supplementation on Markers of Cardiometabolic Risk and Oxidative Stress in Patients with Diabetic Nephropathy: A Randomized Double-blinded Controlled Trial. Iran. J. Kidney Dis. 2018, 12, 156–162. [Google Scholar]
- Baburao Jain, A.; Anand Jain, V. Vitamin E, Its Beneficial Role in Diabetes Mellitus (DM) and Its Complications. J. Clin. Diagn. Res. 2012, 6, 1624–1628. [Google Scholar] [CrossRef]
- Nakhoul, F.M.; Miller-Lotan, R.; Awad, H.; Asleh, R.; Jad, K.; Nakhoul, N.; Asaf, R.; Abu-Saleh, N.; Levy, A.P. Pharmacogenomic effect of vitamin E on kidney structure and function in transgenic mice with the haptoglobin 2-2 genotype and diabetes mellitus. Am. J. Physiol. Renal. Physiol. 2009, 296, F830–F838. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Marco, R.; Codoñer-Franch, P.; Pons Morales, S.; Del Castillo Villaescusa, C.; Boix García, L.; Valls Bellés, V. Oxidant/antioxidant status and hyperfiltration in young patients with type 1 diabetes mellitus. Pediatr. Nephrol. 2009, 24, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Giannini, C.; Lombardo, F.; Currò, F.; Pomilio, M.; Bucciarelli, T.; Chiarelli, F.; Mohn, A. Effects of high-dose vitamin E supplementation on oxidative stress and microalbuminuria in young adult patients with childhood onset type 1 diabetes mellitus. Diabetes Metab. Res. Rev. 2007, 23, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Bursell, S.E.; Clermont, A.C.; Aiello, L.P.; Aiello, L.M.; Schlossman, D.K.; Feener, E.P.; Laffel, L.; King, G.L. High-dose vitamin E supplementation normalizes retinal blood flow and creatinine clearance in patients with type 1 diabetes. Diabetes Care 1999, 22, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, Y.; Khademvatani, K.; Rahimi, B.; Khoshfetrat, M.; Arjmand, N.; Seyyed-Mohammadzad, M.H. Short-Term High-Dose Vitamin E to Prevent Contrast Medium-Induced Acute Kidney Injury in Patients With Chronic Kidney Disease Undergoing Elective Coronary Angiography: A Randomized Placebo-Controlled Trial. J. Am. Heart Assoc. 2016, 5, e002919. [Google Scholar] [CrossRef]
- Khatami, P.G.; Soleimani, A.; Sharifi, N.; Aghadavod, E.; Asemi, Z. The effects of high-dose vitamin E supplementation on biomarkers of kidney injury, inflammation, and oxidative stress in patients with diabetic nephropathy: A randomized, double-blind, placebo-controlled trial. J. Clin. Lipidol. 2016, 10, 922–929. [Google Scholar] [CrossRef]
- Piarulli, F.; Sartore, G.; Ceriello, A.; Ragazzi, E.; Reitano, R.; Nollino, L.; Cosma, C.; Fedele, D.; Lapolla, A. Relationship between glyco-oxidation, antioxidant status and microalbuminuria in type 2 diabetic patients. Diabetologia 2009, 52, 1419–1425. [Google Scholar] [CrossRef] [Green Version]
- Nitti, M.; Furfaro, A.L.; Patriarca, S.; Balbis, E.; Domenicotti, C.; Cottalasso, D.; Pronzato, M.A.; Marinari, U.M.; Traverso, N. Human mesangial cells resist glycoxidative stress through an antioxidant response. Int. J. Mol. Med. 2011, 27, 213–219. [Google Scholar] [CrossRef]
- Monami, M.; Cignarelli, A.; Pinto, S.; D’Onofrio, L.; Milluzzo, A.; Miccoli, R.; Penno, G.; Mannucci, E. Alpha-Tocopherol and contrast-induced nephropathy: A meta-analysis of randomized controlled trials. Int. J. Vitam. Nutr. Res. 2019, 1–9. [Google Scholar] [CrossRef]
- Hayashi, D.; Yagi, K.; Song, C.; Ueda, S.; Yamanoue, M.; Topham, M.; Suzaki, T.; Saito, N.; Emoto, N.; Shirai, Y. Diacylglycerol Kinase alpha is Involved in the Vitamin E-Induced Amelioration of Diabetic Nephropathy in Mice. Sci. Rep. 2017, 7, 2597. [Google Scholar] [CrossRef]
- Kuhad, A.; Chopra, K. Attenuation of diabetic nephropathy by tocotrienol: Involvement of NFκB signaling pathway. Life Sci. 2009, 84, 296–301. [Google Scholar] [CrossRef]
- Modi, J.; Modi, P.; Pal, B.; Bansal, J.; Kumar, S.; Nagarajan, R.; Saifee, Y. Role of Vitamin C and E supplementation in reduction of serum level of renal injury marker following shock wave lithotripsy: Prospective single centre experience. Urol. Ann. 2015, 7, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Park, N.Y.; Park, S.K.; Lim, Y. Long-term dietary antioxidant cocktail supplementation effectively reduces renal inflammation in diabetic mice. Br. J. Nutr. 2011, 106, 1514–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özkaya, D.; Naziroğlu, M.; Armağan, A.; Demirel, A.; Köroglu, B.K.; Çolakoğlu, N.; Kükner, A.; Sönmez, T.T. Dietary vitamin C and E modulates oxidative stress induced-kidney and lens injury in diabetic aged male rats through modulating glucose homeostasis and antioxidant systems. Cell Biochem. Funct. 2011, 29, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, W.; Jia, Q.; Feng, Z.; Guo, J.; Han, X.; Liu, Y.; Shang, H.; Wang, Y.; Liu, W.J. High Dose Vitamin E Attenuates Diabetic Nephropathy via Alleviation of Autophagic Stress. Front. Physiol. 2019, 9, 1939. [Google Scholar] [CrossRef] [Green Version]
- Coronel, I.; Arellano-Mendoza, M.G.; del Valle-Mondragon, L.; Vargas-Robles, H.; Castorena-Torres, F.; Romo, E.; Rios, A.; Escalante, B. L-arginine and antioxidant diet supplementation partially restores nitric oxide-dependent regulation of phenylephrine renal vasoconstriction in diabetics rats. J. Ren. Nutr. 2010, 20, 158–168. [Google Scholar] [CrossRef]
- Devaraj, S.; Jialal, I. Alpha-tocopherol decreases interleukin-1 beta release from activated human monocytes by inhibition of 5-lipoxygenase. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1125–1133. [Google Scholar] [CrossRef]
- Fukunaga-Takenaka, R.; Shirai, Y.; Yagi, K.; Adachi, N.; Sakai, N.; Merino, E.; Merida, I.; Saito, N. Importance of chroman ring and tyrosine phosphorylation in the subtype-specific translocation and activation of diacylglycerol kinase alpha by D-alpha-tocopherol. Genes Cells 2005, 10, 311–319. [Google Scholar] [CrossRef]
- Teupser, D.; Thiery, J.; Seidel, D. Alpha-tocopherol down-regulates scavenger receptor activity in macrophages. Atherosclerosis 1999, 144, 109–115. [Google Scholar] [CrossRef]
- Du, Q.; Luo, Z.C.; Nuyt, A.M.; Audibert, F.; Julien, P.; Wei, S.Q.; Zhang, D.L.; Fraser, W.; Levy, E. Vitamin A and E Nutritional Status in Relation to Leptin, Adiponectin, IGF-I and IGF-II in Early Life—a Birth Cohort Study. Sci. Rep. 2018, 8, 100. [Google Scholar] [CrossRef]
- McMorrow, A.M.; Connaughton, R.M.; Magalhães, T.R.; McGillicuddy, F.C.; Hughes, M.F.; Cheishvili, D.; Morine, M.J.; Ennis, S.; Healy, M.L.; Roche, E.F.; et al. Personalized Cardio-Metabolic Responses to an Anti-Inflammatory Nutrition Intervention in Obese Adolescents: A Randomized Controlled Crossover Trial. Mol. Nutr. Food Res. 2018, 62, e1701008. [Google Scholar] [CrossRef]
- Peris, E.; Micallef, P.; Paul, A.; Palsdottir, V.; Enejder, A.; Bauzá-Thorbrügge, M.; Olofsson, C.S.; Wernstedt Asterholm, I. Antioxidant treatment induces reductive stress associated with mitochondrial dysfunction in adipocytes. J. Biol. Chem. 2019, 294, 2340–2352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alcala, M.; Calderon-Dominguez, M.; Serra, D.; Herrero, L.; Ramos, M.P.; Viana, M. Short-term vitamin E treatment impairs reactive oxygen species signaling required for adipose tissue expansion, resulting in fatty liver and insulin resistance in obese mice. PLoS ONE 2017, 12, e0186579. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.; Ramalingam, L.; Menikdiwela, K.; Scoggin, S.; Shen, C.L.; Tomison, M.D.; Kaur, G.; Dufour, J.M.; Chung, E.; Kalupahana, N.S.; et al. Effects of delta-tocotrienol on obesity-related adipocyte hypertrophy, inflammation and hepatic steatosis in high-fat-fed mice. J. Nutr. Biochem. 2017, 48, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Tanaka-Yachi, R.; Takahashi-Muto, C.; Adachi, K.; Tanimura, Y.; Aoki, Y.; Koike, T.; Kiyose, C. Promoting Effect of α-Tocopherol on Beige Adipocyte Differentiation in 3T3-L1 Cells and Rat White Adipose Tissue. J. Oleo Sci. 2017, 66, 171–179. [Google Scholar] [CrossRef]
- Tanaka-Yachi, R.; Shirasaki, M.; Otsu, R.; Takahashi-Muto, C.; Inoue, H.; Aoki, Y.; Koike, T.; Kiyose, C. δ-Tocopherol promotes thermogenic gene expression via PGC-1α upregulation in 3T3-L1 cells. Biochem. Biophys. Res. Commun. 2018, 506, 53–59. [Google Scholar] [CrossRef]

| Authors | Year | Study Design | Population | Results |
|---|---|---|---|---|
| Mune et al. [22] | 2018 | Prospective | Diabetic and non-diabetic patients with ESRD | Amelioration of endothelial function in diabetic subjects |
| Bolignano et al. [24] | 2017 | Meta-analysis | Type 1 and type 2 diabetes with DKD | Reduction of albuminuria; no effects on GFR |
| Gaede et al. [25] | 2001 | RCT | Type 2 diabetes with DKD | Reduction of albuminuria |
| Tan et al. [26] | 2018 | RCT | Type 2 diabetes with DKD | Reduction of serum creatinine; no effects on proteinuria |
| Aghadavod et al. [28] | 2018 | RCT | Type 1 and type 2 diabetes with DKD | Positive effects on lipid profile; no evaluation on renal function |
| Baburao et al. [29] | 2012 | n-RCT | Type 1 and type 2 diabetes with or without DKD and/or other vascular complications | Positive effects on the development and progression of vascular complications |
| Giannini et al. [32] | 2007 | RCT | Type 1 diabetes with microalbuminuria | Positive effects on oxidative stress; no effects on albuminuria |
| Bursell et al. [33] | 1999 | RCT | Type 1 diabetes | Positive effects on creatinine clearance |
| Rezaei et al. [34] | 2016 | RCT | Diabetic and non-diabetic patients with chronic kidney disease undergoing contrast medium administration | Reduced incidence of contrast-medium induced kidney injury |
| Khatami et al. [35] | 2016 | RCT | Type 2 diabetes with DKD | Reduction of proteinuria, oxidative and inflammatory markers |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Vincenzo, A.; Tana, C.; El Hadi, H.; Pagano, C.; Vettor, R.; Rossato, M. Antioxidant, Anti-Inflammatory, and Metabolic Properties of Tocopherols and Tocotrienols: Clinical Implications for Vitamin E Supplementation in Diabetic Kidney Disease. Int. J. Mol. Sci. 2019, 20, 5101. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20205101
Di Vincenzo A, Tana C, El Hadi H, Pagano C, Vettor R, Rossato M. Antioxidant, Anti-Inflammatory, and Metabolic Properties of Tocopherols and Tocotrienols: Clinical Implications for Vitamin E Supplementation in Diabetic Kidney Disease. International Journal of Molecular Sciences. 2019; 20(20):5101. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20205101
Chicago/Turabian StyleDi Vincenzo, Angelo, Claudio Tana, Hamza El Hadi, Claudio Pagano, Roberto Vettor, and Marco Rossato. 2019. "Antioxidant, Anti-Inflammatory, and Metabolic Properties of Tocopherols and Tocotrienols: Clinical Implications for Vitamin E Supplementation in Diabetic Kidney Disease" International Journal of Molecular Sciences 20, no. 20: 5101. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20205101