The Endocannabinoid System in Pediatric Inflammatory and Immune Diseases
Abstract
:1. Introduction
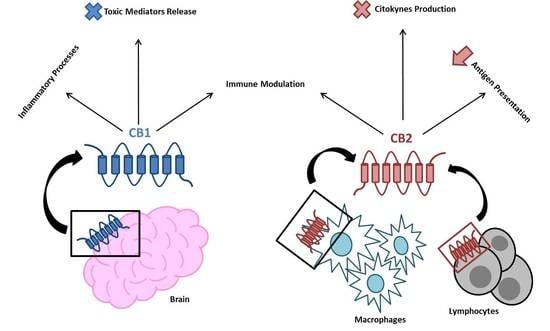
2. The EC System in Inflammation and the Immune Response
3. The EC System in Immune Thrombocytopenia
4. The EC System in Juvenile Idiopathic Arthritis
5. The EC System in Inflammatory Bowel Disease and Celiac Disease
6. The EC System in Obesity and Fatty Liver Disease
7. The EC System in Neuroinflammatory Diseases
8. The EC System in Type 1 Diabetes Mellitus
9. Conclusions
Funding
Conflicts of Interest
References
- Guzman, M. Cannabinoids: Potential anticancer agents. Nat. Rev. Cancer 2003, 3, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Freund, T.F.; Katona, I.; Piomelli, D. Role of endogenous cannabinoids in synaptic signaling. Physiol. Rev. 2003, 83, 1017–1066. [Google Scholar] [CrossRef] [PubMed]
- Farquhar-Smith, W.P.; Egertova, M.; Bradbury, E.J.; McMahon, S.B.; Rice, A.S.; Elphick, M.R. Cannabinoid CB(1) receptor expression in rat spinal cord. Mol. Cell. Neurosci. 2000, 15, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Piomelli, D. The molecular logic of endocannabinoid signalling. Nat. Rev. Neurosci. 2003, 4, 873–884. [Google Scholar] [CrossRef]
- Fride, E. The endocannabinoid-CB receptor system: Importance for development and in pediatric disease. Neuro. Endocrinol. Lett. 2004, 25, 24–30. [Google Scholar]
- Zhang, H.; He, S.; Hu, Y.; Zheng, H. Antagonism of cannabinoid receptor 1 attenuates the anti-inflammatory effects of electroacupuncture in a rodent model of migraine. Acupunct. Med. 2016, 34, 463–470. [Google Scholar] [CrossRef]
- Sipe, J.C.; Arbour, N.; Gerber, A.; Beutler, E. Reduced endocannabinoid immune modulation by a common cannabinoid 2 (CB2) receptor gene polymorphism: Possible risk for autoimmune disorders. J. Leukoc. Biol. 2005, 78, 231–238. [Google Scholar] [CrossRef]
- Donvito, G.; Nass, S.R.; Wilkerson, J.L.; Curry, Z.A.; Schurman, L.D.; Kinsey, S.G.; Lichtman, A.H. The Endogenous Cannabinoid System: A Budding Source of Targets for Treating Inflammatory and Neuropathic Pain. Neuropsychopharmacology 2018, 43, 52–79. [Google Scholar] [CrossRef]
- Boyman, O.; Purton, J.F.; Surh, C.D.; Sprent, J. Cytokines and T-cell homeostasis. Curr. Opin. Immunol. 2007, 19, 320–326. [Google Scholar] [CrossRef]
- Liu, X.; Fang, L.; Guo, T.B.; Mei, H.; Zhang, J.Z. Drug targets in the cytokine universe for autoimmune disease. Trends Immunol. 2013, 34, 120–128. [Google Scholar] [CrossRef]
- Moudgil, K.D.; Choubey, D. Cytokines in autoimmunity: Role in induction, regulation, and treatment. J. Interferon Cytokine Res. 2011, 31, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Lee, S.T.; Lin, W.W. Effects of cannabinoids on LPS-stimulated inflammatory mediator release from macrophages: Involvement of eicosanoids. J. Cell. Biochem 2001, 81, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.W.; Cabral, G.A. Cannabinoid-induced immune suppression and modulation of antigen-presenting cells. J. Neuroimmune Pharmacol. 2006, 1, 50–64. [Google Scholar] [CrossRef] [PubMed]
- McCoy, K.L.; Gainey, D.; Cabral, G.A. delta 9-Tetrahydrocannabinol modulates antigen processing by macrophages. J. Pharmacol. Exp. Ther. 1995, 273, 1216–1223. [Google Scholar]
- Chouinard, F.; Lefebvre, J.S.; Navarro, P.; Bouchard, L.; Ferland, C.; Lalancette-Hebert, M.; Marsolais, D.; Laviolette, M.; Flamand, N. The endocannabinoid 2-arachidonoyl-glycerol activates human neutrophils: Critical role of its hydrolysis and de novo leukotriene B4 biosynthesis. J. Immunol. 2011, 186, 3188–3196. [Google Scholar] [CrossRef] [PubMed]
- Sardinha, J.; Kelly, M.E.; Zhou, J.; Lehmann, C. Experimental cannabinoid 2 receptor-mediated immune modulation in sepsis. Mediators Inflamm. 2014, 2014, 978678. [Google Scholar] [CrossRef] [PubMed]
- Rockwell, C.E.; Raman, P.; Kaplan, B.L.; Kaminski, N.E. A COX-2 metabolite of the endogenous cannabinoid, 2-arachidonyl glycerol, mediates suppression of IL-2 secretion in activated Jurkat T cells. Biochem. Pharmacol. 2008, 76, 353–361. [Google Scholar] [CrossRef]
- Carayon, P.; Marchand, J.; Dussossoy, D.; Derocq, J.M.; Jbilo, O.; Bord, A.; Bouaboula, M.; Galiegue, S.; Mondiere, P.; Penarier, G.; et al. Modulation and functional involvement of CB2 peripheral cannabinoid receptors during B-cell differentiation. Blood 1998, 92, 3605–3615. [Google Scholar] [CrossRef]
- Klein, T.W.; Lane, B.; Newton, C.A.; Friedman, H. The cannabinoid system and cytokine network. Proc. Soc. Exp. Biol Med. 2000, 225, 1–8. [Google Scholar] [CrossRef]
- Sugamura, K.; Sugiyama, S.; Nozaki, T.; Matsuzawa, Y.; Izumiya, Y.; Miyata, K.; Nakayama, M.; Kaikita, K.; Obata, T.; Takeya, M.; et al. Activated endocannabinoid system in coronary artery disease and antiinflammatory effects of cannabinoid 1 receptor blockade on macrophages. Circulation 2009, 119, 28–36. [Google Scholar] [CrossRef]
- Prasanna, S.J.; Gopalakrishnan, D.; Shankar, S.R.; Vasandan, A.B. Pro-inflammatory cytokines, IFNgamma and TNFalpha, influence immune properties of human bone marrow and Wharton jelly mesenchymal stem cells differentially. PLoS ONE 2010, 5, e9016. [Google Scholar] [CrossRef] [PubMed]
- Keating, A. Mesenchymal stromal cells: New directions. Cell Stem Cell 2012, 10, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Bernardo, M.E.; Bellini, G.; Luongo, L.; Conforti, A.; Manzo, I.; Guida, F.; Cristino, L.; Imperatore, R.; Petrosino, S.; et al. The cannabinoid receptor type 2 as mediator of mesenchymal stromal cell immunosuppressive properties. PLoS ONE 2013, 8, e80022. [Google Scholar] [CrossRef] [PubMed]
- Quach, M.E.; Dragovich, M.A.; Chen, W.; Syed, A.K.; Cao, W.; Liang, X.; Deng, W.; De Meyer, S.F.; Zhu, G.; Peng, J.; et al. Fc-independent immune thrombocytopenia via mechanomolecular signaling in platelets. Blood 2018, 131, 787–796. [Google Scholar] [CrossRef]
- Rossi, F.; Mancusi, S.; Bellini, G.; Roberti, D.; Punzo, F.; Vetrella, S.; Matarese, S.M.; Nobili, B.; Maione, S.; Perrotta, S. CNR2 functional variant (Q63R) influences childhood immune thrombocytopenic purpura. Haematologica 2011, 96, 1883–1885. [Google Scholar] [CrossRef]
- Ezzat, D.A.; Hammam, A.A.; El-Malah, W.M.; Khattab, R.A.; Mangoud, E.M. Role of Cannabinoid CB2 Receptor Gene (CNR2) Polymorphism in Children with Immune Thrombocytopenic Purpura in Beni-Suef Governorate in Egypt. Egypt J. Immunol. 2017, 24, 57–66. [Google Scholar]
- Mahmoud Gouda, H.; Mohamed Kamel, N.R. Cannabinoid CB2 receptor gene (CNR2) polymorphism is associated with chronic childhood immune thrombocytopenia in Egypt. Blood Coagul. Fibrinolysis 2013, 24, 247–251. [Google Scholar] [CrossRef]
- Audia, S.; Mahevas, M.; Samson, M.; Godeau, B.; Bonnotte, B. Pathogenesis of immune thrombocytopenia. Autoimmun Rev. 2017, 16, 620–632. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, Y.; Ruan, M.; Shi, J. The Levels of T Lymphocyte Subsets in Immune Thrombocytopenia Associated with Anti-GPIIb/IIIa- and/or Anti-GPIbalpha-Mediated Responses Are Differentially Sensitive to Dexamethasone. Acta Haematol. 2018, 140, 60–66. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, H.; Poon, M.C.; Han, Z.; Gu, D.; Xu, M.; Jia, H.; Yang, R.; Han, Z.C. Abnormality of CD4(+)CD25(+) regulatory T cells in idiopathic thrombocytopenic purpura. Eur J. Haematol. 2007, 78, 139–143. [Google Scholar] [CrossRef]
- Yazdanbakhsh, K. Imbalanced immune homeostasis in immune thrombocytopenia. Semin. Hematol. 2016, 53, S16–S19. [Google Scholar] [CrossRef] [PubMed]
- Ogawara, H.; Handa, H.; Morita, K.; Hayakawa, M.; Kojima, J.; Amagai, H.; Tsumita, Y.; Kaneko, Y.; Tsukamoto, N.; Nojima, Y.; et al. High Th1/Th2 ratio in patients with chronic idiopathic thrombocytopenic purpura. Eur. J. Haematol. 2003, 71, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Perera, M.; Garrido, T. Advances in the pathophysiology of primary immune thrombocytopenia. Hematology 2017, 22, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Li, M.O.; Flavell, R.A. Contextual regulation of inflammation: A duet by transforming growth factor-beta and interleukin-10. Immunity 2008, 28, 468–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geginat, J.; Larghi, P.; Paroni, M.; Nizzoli, G.; Penatti, A.; Pagani, M.; Gagliani, N.; Meroni, P.; Abrignani, S.; Flavell, R.A. The light and the dark sides of Interleukin-10 in immune-mediated diseases and cancer. Cytokine Growth Factor Rev. 2016, 30, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Yang, Y.; Xiang, X.; Wu, Y. Relationship between the IL-10 (-1082 A/G) polymorphism and the risk of immune/idiopathic thrombocytopenic purpura: A meta-analysis. Cytokine 2019, 125, 154820. [Google Scholar] [CrossRef]
- Saitoh, T.; Kasamatsu, T.; Inoue, M.; Mitsui, T.; Koiso, H.; Yokohama, A.; Handa, H.; Matsushima, T.; Tsukamoto, N.; Karasawa, M.; et al. Interleukin-10 gene polymorphism reflects the severity of chronic immune thrombocytopenia in Japanese patients. Int J. Lab. Hematol. 2011, 33, 526–532. [Google Scholar] [CrossRef]
- Tesse, R.; Del Vecchio, G.C.; De Mattia, D.; Sangerardi, M.; Valente, F.; Giordano, P. Association of interleukin-(IL)10 haplotypes and serum IL-10 levels in the progression of childhood immune thrombocytopenic purpura. Gene 2012, 505, 53–56. [Google Scholar] [CrossRef]
- El Ghannam, D.; Fawzy, I.M.; Azmy, E.; Hakim, H.; Eid, I. Relation of interleukin-10 Promoter Polymorphisms to Adult Chronic Immune Thrombocytopenic Purpura in a Cohort of Egyptian Population. Immunol. Invest. 2015, 44, 616–626. [Google Scholar] [CrossRef]
- Soliman, M.A.; Helwa, M.A.; Fath-Allah, S.K.; El-Hawy, M.A.; Badr, H.S.; Barseem, N.F. IL-10 polymorphisms and T-cell subsets could affect the clinical presentation and outcome of childhood immune thrombocytopenia in Egyptian population. APMIS 2018, 126, 380–388. [Google Scholar] [CrossRef]
- Tahamtan, A.; Tavakoli-Yaraki, M.; Shadab, A.; Rezaei, F.; Marashi, S.M.; Shokri, F.; Mokhatri-Azad, T.; Salimi, V. The Role of Cannabinoid Receptor 1 in the Immunopathology of Respiratory Syncytial Virus. Viral Immunol. 2018, 31, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.H.; Meissler, J.J.; Fan, X.; Yu, D.; Adler, M.W.; Eisenstein, T.K. A CB2-Selective Cannabinoid Suppresses T-Cell Activities and Increases Tregs and IL-10. J. Neuroimmune Pharmacol. 2015, 10, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Chiu, S.M.; Motan, D.A.; Zhang, Z.; Chen, L.; Ji, H.L.; Tse, H.F.; Fu, Q.L.; Lian, Q. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis. 2016, 7, e2062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Simon, J.A.; Tabera, S.; Sarasquete, M.E.; Diez-Campelo, M.; Canchado, J.; Sanchez-Abarca, L.I.; Blanco, B.; Alberca, I.; Herrero-Sanchez, C.; Canizo, C.; et al. Mesenchymal stem cells are functionally abnormal in patients with immune thrombocytopenic purpura. Cytotherapy 2009, 11, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; Zhu, X.L.; Xue, J.; Liu, X.; Long Zheng, X.; Chang, Y.J.; Liu, K.Y.; Huang, X.J.; Zhang, X.H. Integrated mRNA and miRNA profiling revealed deregulation of cellular stress response in bone marrow mesenchymal stem cells derived from patients with immune thrombocytopenia. Funct. Integr. Genomics 2018, 18, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Tortora, C.; Palumbo, G.; Punzo, F.; Argenziano, M.; Casale, M.; Di Paola, A.; Locatelli, F.; Perrotta, S. CB2 Receptor Stimulation and Dexamethasone Restore the Anti-Inflammatory and Immune-Regulatory Properties of Mesenchymal Stromal Cells of Children with Immune Thrombocytopenia. Int. J. Mol. Sci. 2019, 20, 1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maccarrone, M.; Del Principe, D.; Finazzi-Agro, A. Endocannabinoids: New physiological (co-)agonists of human platelets. Thromb. Haemost. 2002, 88, 165–166. [Google Scholar]
- Catani, M.V.; Gasperi, V.; Evangelista, D.; Finazzi Agro, A.; Avigliano, L.; Maccarrone, M. Anandamide extends platelets survival through CB(1)-dependent Akt signaling. Cell. Mol. Life Sci. 2010, 67, 601–610. [Google Scholar] [CrossRef]
- Catani, M.V.; Fezza, F.; Baldassarri, S.; Gasperi, V.; Bertoni, A.; Pasquariello, N.; Finazzi-Agro, A.; Sinigaglia, F.; Avigliano, L.; Maccarrone, M. Expression of the endocannabinoid system in the bi-potential HEL cell line: Commitment to the megakaryoblastic lineage by 2-arachidonoylglycerol. J. Mol. Med. 2009, 87, 65–74. [Google Scholar] [CrossRef]
- Gasperi, V.; Avigliano, L.; Evangelista, D.; Oddi, S.; Chiurchiu, V.; Lanuti, M.; Maccarrone, M.; Valeria Catani, M. 2-Arachidonoylglycerol enhances platelet formation from human megakaryoblasts. Cell cycle 2014, 13, 3938–3947. [Google Scholar] [CrossRef] [Green Version]
- Marshall, A.; Gupta, K.; Pazirandeh, M.; Bonafede, M.; McMorrow, D. Treatment patterns and economic outcomes in patients with juvenile idiopathic arthritis. Clinicoecon Outcomes Res. 2019, 11, 361–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, K.; Takayanagi, H. Osteoclasts, rheumatoid arthritis, and osteoimmunology. Curr. Opin. Rheumatol. 2006, 18, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.; Pearson, R.G.; Kurian, N.; Latif, M.L.; Garle, M.J.; Barrett, D.A.; Kendall, D.A.; Scammell, B.E.; Reeve, A.J.; Chapman, V. Characterisation of the cannabinoid receptor system in synovial tissue and fluid in patients with osteoarthritis and rheumatoid arthritis. Arthritis Res. Ther. 2008, 10, R43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDougall, J.J.; Muley, M.M.; Philpott, H.T.; Reid, A.; Krustev, E. Early blockade of joint inflammation with a fatty acid amide hydrolase inhibitor decreases end-stage osteoarthritis pain and peripheral neuropathy in mice. Arthritis Res. Ther. 2017, 19, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falconer, J.; Murphy, A.N.; Young, S.P.; Clark, A.R.; Tiziani, S.; Guma, M.; Buckley, C.D. Review: Synovial Cell Metabolism and Chronic Inflammation in Rheumatoid Arthritis. Arthritis Rheumatol. 2018, 70, 984–999. [Google Scholar] [CrossRef]
- Fechtner, S.C.; Singh, A.K.; Ahmed, S. Role of cannabinoid receptor 2 in mediating interleukin-1beta-induced inflammation in rheumatoid arthritis synovial fibroblasts. Clin. Exp. Rheumatol. 2019. [Google Scholar]
- Bai, J.; Ge, G.; Wang, Y.; Zhang, W.; Wang, Q.; Wang, W.; Guo, X.; Yu, B.; Xu, Y.; Yang, H.; et al. A selective CB2 agonist protects against the inflammatory response and joint destruction in collagen-induced arthritis mice. Biomed. Pharmacother. 2019, 116, 109025. [Google Scholar] [CrossRef]
- Idris, A.I.; van ’t Hof, R.J.; Greig, I.R.; Ridge, S.A.; Baker, D.; Ross, R.A.; Ralston, S.H. Regulation of bone mass, bone loss and osteoclast activity by cannabinoid receptors. Nat. Med. 2005, 11, 774–779. [Google Scholar] [CrossRef] [Green Version]
- Ofek, O.; Attar-Namdar, M.; Kram, V.; Dvir-Ginzberg, M.; Mechoulam, R.; Zimmer, A.; Frenkel, B.; Shohami, E.; Bab, I. CB2 cannabinoid receptor targets mitogenic Gi protein-cyclin D1 axis in osteoblasts. J. Bone Miner. Res. 2011, 26, 308–316. [Google Scholar] [CrossRef] [Green Version]
- Rossi, F.; Tortora, C.; Punzo, F.; Bellini, G.; Argenziano, M.; Di Paola, A.; Torella, M.; Perrotta, S. The Endocannabinoid/Endovanilloid System in Bone: From Osteoporosis to Osteosarcoma. Int J. Mol. Sci. 2019, 20, 1919. [Google Scholar] [CrossRef] [Green Version]
- Rossi, F.; Bellini, G.; Tortora, C.; Bernardo, M.E.; Luongo, L.; Conforti, A.; Starc, N.; Manzo, I.; Nobili, B.; Locatelli, F.; et al. CB(2) and TRPV(1) receptors oppositely modulate in vitro human osteoblast activity. Pharmacol. Res. 2015, 99, 194–201. [Google Scholar] [CrossRef]
- Tyrovola, J.B.; Spyropoulos, M.N.; Makou, M.; Perrea, D. Root resorption and the OPG/RANKL/RANK system: A mini review. J. Oral Sci. 2008, 50, 367–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geusens, P.P.; Landewe, R.B.; Garnero, P.; Chen, D.; Dunstan, C.R.; Lems, W.F.; Stinissen, P.; van der Heijde, D.M.; van der Linden, S.; Boers, M. The ratio of circulating osteoprotegerin to RANKL in early rheumatoid arthritis predicts later joint destruction. Arthritis Rheum. 2006, 54, 1772–1777. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Yu, B.; Bai, J.; Wang, X.; Guo, X.; Liu, Y.; Lin, J.; Hu, S.; Zhang, W.; Tao, Y.; et al. Cannabinoid Receptor 2 Agonist Prevents Local and Systemic Inflammatory Bone Destruction in Rheumatoid Arthritis. J. Bone Miner. Res. 2019, 34, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Bellini, G.; Olivieri, A.N.; Grandone, A.; Alessio, M.; Gicchino, M.F.; Nobili, B.; Perrone, L.; Maione, S.; del Giudice, E.M.; Rossi, F. Association between cannabinoid receptor type 2 Q63R variant and oligo/polyarticular juvenile idiopathic arthritis. Scand. J. Rheumatol. 2015, 44, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Peyrin-Biroulet, L.; Eisenhut, M.; Shin, J.I. IBD immunopathogenesis: A comprehensive review of inflammatory molecules. Autoimmun. Rev. 2017, 16, 416–426. [Google Scholar] [CrossRef]
- Di Marzo, V.; Izzo, A.A. Endocannabinoid overactivity and intestinal inflammation. Gut 2006, 55, 1373–1376. [Google Scholar] [CrossRef] [Green Version]
- D’Argenio, G.; Valenti, M.; Scaglione, G.; Cosenza, V.; Sorrentini, I.; Di Marzo, V. Up-regulation of anandamide levels as an endogenous mechanism and a pharmacological strategy to limit colon inflammation. FASEB J. 2006, 20, 568–570. [Google Scholar] [CrossRef]
- Cencioni, M.T.; Chiurchiu, V.; Catanzaro, G.; Borsellino, G.; Bernardi, G.; Battistini, L.; Maccarrone, M. Anandamide suppresses proliferation and cytokine release from primary human T-lymphocytes mainly via CB2 receptors. PLoS ONE 2010, 5, e8688. [Google Scholar] [CrossRef]
- Massa, F.; Marsicano, G.; Hermann, H.; Cannich, A.; Monory, K.; Cravatt, B.F.; Ferri, G.L.; Sibaev, A.; Storr, M.; Lutz, B. The endogenous cannabinoid system protects against colonic inflammation. J. Clin. Invest. 2004, 113, 1202–1209. [Google Scholar] [CrossRef] [Green Version]
- Alhouayek, M.; Lambert, D.M.; Delzenne, N.M.; Cani, P.D.; Muccioli, G.G. Increasing endogenous 2-arachidonoylglycerol levels counteracts colitis and related systemic inflammation. FASEB J. 2011, 25, 2711–2721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, K.; Rooney, N.; Feeney, M.; Tate, J.; Robertson, D.; Welham, M.; Ward, S. Differential expression of cannabinoid receptors in the human colon: Cannabinoids promote epithelial wound healing. Gastroenterology 2005, 129, 437–453. [Google Scholar] [CrossRef] [PubMed]
- Ihenetu, K.; Molleman, A.; Parsons, M.E.; Whelan, C.J. Inhibition of interleukin-8 release in the human colonic epithelial cell line HT-29 by cannabinoids. Eur. J. Pharmacol. 2003, 458, 207–215. [Google Scholar] [CrossRef]
- Leinwand, K.L.; Jones, A.A.; Huang, R.H.; Jedlicka, P.; Kao, D.J.; de Zoeten, E.F.; Ghosh, S.; Moaddel, R.; Wehkamp, J.; Ostaff, M.J.; et al. Cannabinoid Receptor-2 Ameliorates Inflammation in Murine Model of Crohn’s Disease. J. Crohns Colitis 2017, 11, 1369–1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uranga, J.A.; Vera, G.; Abalo, R. Cannabinoid pharmacology and therapy in gut disorders. Biochem. Pharmacol. 2018, 157, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Yonal, O.; Eren, F.; Yilmaz, Y.; Atug, O.; Over, H.H. No association between the functional cannabinoid receptor type 2 Q63R variants and inflammatory bowel disease in Turkish subjects. Turk. J. Gastroenterol. 2014, 25, 639–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strisciuglio, C.; Bellini, G.; Miele, E.; Martinelli, M.; Cenni, S.; Tortora, C.; Tolone, C.; Miraglia Del Giudice, E.; Rossi, F. Cannabinoid Receptor 2 Functional Variant Contributes to the Risk for Pediatric Inflammatory Bowel Disease. J. Clin. Gastroenterol. 2018, 52, e37–e43. [Google Scholar] [CrossRef]
- Basu, D.; Lopez, I.; Kulkarni, A.; Sellin, J.H. Impact of race and ethnicity on inflammatory bowel disease. Am. J. Gastroenterol. 2005, 100, 2254–2261. [Google Scholar] [CrossRef]
- Tye-Din, J.A.; Galipeau, H.J.; Agardh, D. Celiac Disease: A Review of Current Concepts in Pathogenesis, Prevention, and Novel Therapies. Front. Pediatr. 2018, 6, 350. [Google Scholar] [CrossRef]
- Rossi, F.; Bellini, G.; Tolone, C.; Luongo, L.; Mancusi, S.; Papparella, A.; Sturgeon, C.; Fasano, A.; Nobili, B.; Perrone, L.; et al. The cannabinoid receptor type 2 Q63R variant increases the risk of celiac disease: Implication for a novel molecular biomarker and future therapeutic intervention. Pharmacol Res. 2012, 66, 88–94. [Google Scholar] [CrossRef]
- Battista, N.; Di Sabatino, A.; Di Tommaso, M.; Biancheri, P.; Rapino, C.; Vidali, F.; Papadia, C.; Montana, C.; Pasini, A.; Lanzini, A.; et al. Abnormal anandamide metabolism in celiac disease. J. Nutr. Biochem. 2012, 23, 1245–1248. [Google Scholar] [CrossRef] [PubMed]
- D’Argenio, G.; Petrosino, S.; Gianfrani, C.; Valenti, M.; Scaglione, G.; Grandone, I.; Nigam, S.; Sorrentini, I.; Mazzarella, G.; Di Marzo, V. Overactivity of the intestinal endocannabinoid system in celiac disease and in methotrexate-treated rats. J. Mol. Med. 2007, 85, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, L.; Ciacci, C.; Ricciardelli, I.; Vacca, L.; Raia, V.; Auricchio, S.; Picard, J.; Osman, M.; Quaratino, S.; Londei, M. Association between innate response to gliadin and activation of pathogenic T cells in coeliac disease. Lancet 2003, 362, 30–37. [Google Scholar] [CrossRef]
- Battista, N.; Di Sabatino, A.; Di Tommaso, M.; Biancheri, P.; Rapino, C.; Giuffrida, P.; Papadia, C.; Montana, C.; Pasini, A.; Vanoli, A.; et al. Altered expression of type-1 and type-2 cannabinoid receptors in celiac disease. PLoS ONE 2013, 8, e62078. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Kelly, A.S. Review of Childhood Obesity: From Epidemiology, Etiology, and Comorbidities to Clinical Assessment and Treatment. Mayo Clin. Proc. 2017, 92, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.S.; Clement, K.; Baur, L.A.; Tordjman, J. Obesity and low-grade inflammation: A paediatric perspective. Obes. Rev. 2010, 11, 118–126. [Google Scholar] [CrossRef]
- Maffeis, C.; Silvagni, D.; Bonadonna, R.; Grezzani, A.; Banzato, C.; Tato, L. Fat cell size, insulin sensitivity, and inflammation in obese children. J. Pediatr. 2007, 151, 647–652. [Google Scholar] [CrossRef]
- Valle Jimenez, M.; Estepa, R.M.; Camacho, R.M.; Estrada, R.C.; Luna, F.G.; Guitarte, F.B. Endothelial dysfunction is related to insulin resistance and inflammatory biomarker levels in obese prepubertal children. Eur. J. Endocrinol. 2007, 156, 497–502. [Google Scholar] [CrossRef]
- Caballero, A.E.; Bousquet-Santos, K.; Robles-Osorio, L.; Montagnani, V.; Soodini, G.; Porramatikul, S.; Hamdy, O.; Nobrega, A.C.; Horton, E.S. Overweight Latino children and adolescents have marked endothelial dysfunction and subclinical vascular inflammation in association with excess body fat and insulin resistance. Diabetes Care 2008, 31, 576–582. [Google Scholar] [CrossRef] [Green Version]
- Watkins, B.A.; Kim, J. The endocannabinoid system: Directing eating behavior and macronutrient metabolism. Front. Psychol. 2014, 5, 1506. [Google Scholar] [CrossRef]
- Rossi, F.; Punzo, F.; Umano, G.R.; Argenziano, M.; Miraglia Del Giudice, E. Role of Cannabinoids in Obesity. Int. J. Mol. Sci. 2018, 19, 2690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz de Azua, I.; Mancini, G.; Srivastava, R.K.; Rey, A.A.; Cardinal, P.; Tedesco, L.; Zingaretti, C.M.; Sassmann, A.; Quarta, C.; Schwitter, C.; et al. Adipocyte cannabinoid receptor CB1 regulates energy homeostasis and alternatively activated macrophages. J. Clin. Invest. 2017, 127, 4148–4162. [Google Scholar] [CrossRef] [PubMed]
- Alshaarawy, O.; Kurjan, E.; Truong, N.; Olson, L.K. Diet-Induced Obesity in Cannabinoid-2 Receptor Knockout Mice and Cannabinoid Receptor 1/2 Double-Knockout Mice. Obesity (Silver Spring) 2019, 27, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Mechoulam, R. Is lipid signaling through cannabinoid 2 receptors part of a protective system? Prog. Lipid Res. 2011, 50, 193–211. [Google Scholar] [CrossRef] [Green Version]
- Agudo, J.; Martin, M.; Roca, C.; Molas, M.; Bura, A.S.; Zimmer, A.; Bosch, F.; Maldonado, R. Deficiency of CB2 cannabinoid receptor in mice improves insulin sensitivity but increases food intake and obesity with age. Diabetologia 2010, 53, 2629–2640. [Google Scholar] [CrossRef] [Green Version]
- Deveaux, V.; Cadoudal, T.; Ichigotani, Y.; Teixeira-Clerc, F.; Louvet, A.; Manin, S.; Nhieu, J.T.; Belot, M.P.; Zimmer, A.; Even, P.; et al. Cannabinoid CB2 receptor potentiates obesity-associated inflammation, insulin resistance and hepatic steatosis. PLoS ONE 2009, 4, e5844. [Google Scholar] [CrossRef]
- Xu, H.; Cheng, C.L.; Chen, M.; Manivannan, A.; Cabay, L.; Pertwee, R.G.; Coutts, A.; Forrester, J.V. Anti-inflammatory property of the cannabinoid receptor-2-selective agonist JWH-133 in a rodent model of autoimmune uveoretinitis. J. Leukoc. Biol. 2007, 82, 532–541. [Google Scholar] [CrossRef]
- Rossi, F.; Bellini, G.; Luongo, L.; Manzo, I.; Tolone, S.; Tortora, C.; Bernardo, M.E.; Grandone, A.; Conforti, A.; Docimo, L.; et al. Cannabinoid Receptor 2 as Antiobesity Target: Inflammation, Fat Storage, and Browning Modulation. J. Clin. Endocrinol. Metab. 2016, 101, 3469–3478. [Google Scholar] [CrossRef] [Green Version]
- Ishiguro, H.; Carpio, O.; Horiuchi, Y.; Shu, A.; Higuchi, S.; Schanz, N.; Benno, R.; Arinami, T.; Onaivi, E.S. A nonsynonymous polymorphism in cannabinoid CB2 receptor gene is associated with eating disorders in humans and food intake is modified in mice by its ligands. Synapse 2010, 64, 92–96. [Google Scholar] [CrossRef]
- Doris, J.M.; Millar, S.A.; Idris, I.; O’Sullivan, S.E. Genetic polymorphisms of the endocannabinoid system in obesity and diabetes. Diabetes. Obes. Metab. 2019, 21, 382–387. [Google Scholar] [CrossRef]
- de Luis, D.; Aller, R.; Izaola, O.; Conde, R.; de la Fuente, B.; Gonzalez Sagrado, M. Genetic variation in the endocannabinoid degrading enzyme fatty acid amide hydrolase (FAAH) and their influence on weight loss and insulin resistance under a high monounsaturated fat hypocaloric diet. J. Diabetes Complications 2013, 27, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Unamuno, X.; Gomez-Ambrosi, J.; Rodriguez, A.; Becerril, S.; Fruhbeck, G.; Catalan, V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur. J. Clin. Invest. 2018, 48, e12997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, F.; Bellini, G.; Alisi, A.; Alterio, A.; Maione, S.; Perrone, L.; Locatelli, F.; Miraglia del Giudice, E.; Nobili, V. Cannabinoid receptor type 2 functional variant influences liver damage in children with non-alcoholic fatty liver disease. PLoS ONE 2012, 7, e42259. [Google Scholar] [CrossRef] [PubMed]
- Bazwinsky-Wutschke, I.; Zipprich, A.; Dehghani, F. Endocannabinoid System in Hepatic Glucose Metabolism, Fatty Liver Disease, and Cirrhosis. Int. J. Mol. Sci. 2019, 20, 2516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van de Velde, F.; Bekaert, M.; Geerts, A.; Hoorens, A.; Batens, A.H.; Samyah, S.; Ouwens, M.; Van Nieuwenhove, Y.; Lapauw, B. Insulin resistance associates with hepatic lobular inflammation in subjects with obesity. Endocr. Connect. 2019, 8, 1294–1301. [Google Scholar] [CrossRef] [Green Version]
- Coppola, N.; Zampino, R.; Bellini, G.; Macera, M.; Marrone, A.; Pisaturo, M.; Boemio, A.; Nobili, B.; Pasquale, G.; Maione, S.; et al. Association between a polymorphism in cannabinoid receptor 2 and severe necroinflammation in patients with chronic hepatitis C. Clin. Gastroenterol. Hepatol. 2014, 12, 334–340. [Google Scholar] [CrossRef]
- Coppola, N.; Zampino, R.; Bellini, G.; Stanzione, M.; Capoluongo, N.; Marrone, A.; Macera, M.; Adinolfi, L.E.; Giudice, E.M.; Gentile, I.; et al. CB2-63 polymorphism and immune-mediated diseases associated with HCV chronic infection. Dig. Liver Dis. 2016, 48, 1364–1369. [Google Scholar] [CrossRef]
- Freeman, L.C.; Ting, J.P. The pathogenic role of the inflammasome in neurodegenerative diseases. J. Neurochem. 2016, 136, 29–38. [Google Scholar] [CrossRef]
- Bjorklund, G.; Saad, K.; Chirumbolo, S.; Kern, J.K.; Geier, D.A.; Geier, M.R.; Urbina, M.A. Immune dysfunction and neuroinflammation in autism spectrum disorder. Acta Neurobiol. Exp. (Wars) 2016, 76, 257–268. [Google Scholar] [CrossRef] [Green Version]
- Terrone, G.; Salamone, A.; Vezzani, A. Inflammation and Epilepsy: Preclinical Findings and Potential Clinical Translation. Curr. Pharm. Des. 2017, 23, 5569–5576. [Google Scholar] [CrossRef]
- Koppel, B.S.; Brust, J.C.; Fife, T.; Bronstein, J.; Youssof, S.; Gronseth, G.; Gloss, D. Systematic review: Efficacy and safety of medical marijuana in selected neurologic disorders: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2014, 82, 1556–1563. [Google Scholar] [CrossRef] [PubMed]
- Hill, T.D.; Cascio, M.G.; Romano, B.; Duncan, M.; Pertwee, R.G.; Williams, C.M.; Whalley, B.J.; Hill, A.J. Cannabidivarin-rich cannabis extracts are anticonvulsant in mouse and rat via a CB1 receptor-independent mechanism. Br. J. Pharmacol. 2013, 170, 679–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rom, S.; Persidsky, Y. Cannabinoid receptor 2: Potential role in immunomodulation and neuroinflammation. J. Neuroimmune Pharmacol. 2013, 8, 608–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastinu, A.; Premoli, M.; Ferrari-Toninelli, G.; Tambaro, S.; Maccarinelli, G.; Memo, M.; Bonini, S.A. Cannabinoids in health and disease: Pharmacological potential in metabolic syndrome and neuroinflammation. Horm Mol. Biol. Clin. Investig. 2018, 36. [Google Scholar] [CrossRef]
- Crunfli, F.; Vrechi, T.A.; Costa, A.P.; Torrao, A.S. Cannabinoid Receptor Type 1 Agonist ACEA Improves Cognitive Deficit on STZ-Induced Neurotoxicity Through Apoptosis Pathway and NO Modulation. Neurotox. Res. 2019, 35, 516–529. [Google Scholar] [CrossRef]
- Zou, Z.; Lu, Y.; Zha, Y.; Yang, H. Endocannabinoid 2-Arachidonoylglycerol Suppresses LPS-Induced Inhibition of A-Type Potassium Channel Currents in Caudate Nucleus Neurons Through CB1 Receptor. J. Mol. Neurosci. 2016, 59, 493–503. [Google Scholar] [CrossRef]
- Albayram, O.; Alferink, J.; Pitsch, J.; Piyanova, A.; Neitzert, K.; Poppensieker, K.; Mauer, D.; Michel, K.; Legler, A.; Becker, A.; et al. Role of CB1 cannabinoid receptors on GABAergic neurons in brain aging. PNAS 2011, 108, 11256–11261. [Google Scholar] [CrossRef] [Green Version]
- Malek, N.; Popiolek-Barczyk, K.; Mika, J.; Przewlocka, B.; Starowicz, K. Anandamide, Acting via CB2 Receptors, Alleviates LPS-Induced Neuroinflammation in Rat Primary Microglial Cultures. Neural Plast. 2015, 2015, 130639. [Google Scholar] [CrossRef] [Green Version]
- Mecha, M.; Carrillo-Salinas, F.J.; Feliu, A.; Mestre, L.; Guaza, C. Microglia activation states and cannabinoid system: Therapeutic implications. Pharmalcol. Ther. 2016, 166, 40–55. [Google Scholar] [CrossRef]
- Tanaka, M.; Yagyu, K.; Sackett, S.; Zhang, Y. Anti-Inflammatory Effects by Pharmacological Inhibition or Knockdown of Fatty Acid Amide Hydrolase in BV2 Microglial Cells. Cells 2019, 8, 491. [Google Scholar] [CrossRef] [Green Version]
- Vrechi, T.A.; Crunfli, F.; Costa, A.P.; Torrao, A.S. Cannabinoid Receptor Type 1 Agonist ACEA Protects Neurons from Death and Attenuates Endoplasmic Reticulum Stress-Related Apoptotic Pathway Signaling. Neurotox. Res. 2018, 33, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Filloux, F.M. Cannabinoids for pediatric epilepsy? Up in smoke or real science? Transl. Pediatr. 2015, 4, 271–282. [Google Scholar] [PubMed]
- Devinsky, O.; Marsh, E.; Friedman, D.; Thiele, E.; Laux, L.; Sullivan, J.; Miller, I.; Flamini, R.; Wilfong, A.; Filloux, F.; et al. Cannabidiol in patients with treatment-resistant epilepsy: An open-label interventional trial. Lancet Neurol. 2016, 15, 270–278. [Google Scholar] [CrossRef]
- Siniscalco, D.; Bradstreet, J.J.; Cirillo, A.; Antonucci, N. The in vitro GcMAF effects on endocannabinoid system transcriptionomics, receptor formation, and cell activity of autism-derived macrophages. J. Neuroinflammation 2014, 11, 78. [Google Scholar] [CrossRef] [Green Version]
- Krzewska, A.; Ben-Skowronek, I. Effect of Associated Autoimmune Diseases on Type 1 Diabetes Mellitus Incidence and Metabolic Control in Children and Adolescents. Biomed. Res. Int. 2016, 2016, 6219730. [Google Scholar] [CrossRef] [Green Version]
- Domingueti, C.P.; Dusse, L.M.; Carvalho, M.; de Sousa, L.P.; Gomes, K.B.; Fernandes, A.P. Diabetes mellitus: The linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J. Diabetes Complications 2016, 30, 738–745. [Google Scholar] [CrossRef]
- Bermudez-Silva, F.J.; Suarez, J.; Baixeras, E.; Cobo, N.; Bautista, D.; Cuesta-Munoz, A.L.; Fuentes, E.; Juan-Pico, P.; Castro, M.J.; Milman, G.; et al. Presence of functional cannabinoid receptors in human endocrine pancreas. Diabetologia 2008, 51, 476–487. [Google Scholar] [CrossRef] [Green Version]
- Horvath, B.; Magid, L.; Mukhopadhyay, P.; Batkai, S.; Rajesh, M.; Park, O.; Tanchian, G.; Gao, R.Y.; Goodfellow, C.E.; Glass, M.; et al. A new cannabinoid CB2 receptor agonist HU-910 attenuates oxidative stress, inflammation and cell death associated with hepatic ischaemia/reperfusion injury. Br. J. Pharmacol. 2012, 165, 2462–2478. [Google Scholar] [CrossRef] [Green Version]
- Zoja, C.; Locatelli, M.; Corna, D.; Villa, S.; Rottoli, D.; Nava, V.; Verde, R.; Piscitelli, F.; Di Marzo, V.; Fingerle, J.; et al. Therapy with a Selective Cannabinoid Receptor Type 2 Agonist Limits Albuminuria and Renal Injury in Mice with Type 2 Diabetic Nephropathy. Nephron 2016, 132, 59–69. [Google Scholar] [CrossRef]
- Kumawat, V.S.; Kaur, G. Therapeutic potential of cannabinoid receptor 2 in the treatment of diabetes mellitus and its complications. Eur. J. Pharmacol. 2019, 862, 172628. [Google Scholar] [CrossRef]
- Vera, G.; Lopez-Miranda, V.; Herradon, E.; Martin, M.I.; Abalo, R. Characterization of cannabinoid-induced relief of neuropathic pain in rat models of type 1 and type 2 diabetes. Pharmacol. Biochem. Behav. 2012, 102, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Bujalska, M. Effect of cannabinoid receptor agonists on streptozotocin-induced hyperalgesia in diabetic neuropathy. Pharmacology 2008, 82, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, O.; Lang, Y.; Idrees, Z.; McGuire, B.E.; Finn, D.P. Impaired cued and spatial learning performance and altered cannabinoid CB(1) receptor functionality in the substantia nigra in a rat model of diabetic neuropathy. Behav. Brain Res. 2016, 303, 61–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Disease | Main Alterations in the EC System |
|---|---|
| Immune thrombocytopenia | Increased risk by CNR2 rs35761398 variant; reduced CB2 receptor expression in Mesenchymal Stromal Cells (MSC) |
| Juvenile idiopathic arthritis | Increased risk by CNR2 rs35761398 variant; presence of EC elements in synovial fluid; inflammation; RANK-L accumulation in joints |
| Inflammatory bowel diseases | Increased risk by CNR2 rs35761398 variant; CB receptors hyper-expression; increased AEA levels |
| Celiac Disease | Increased risk by CNR2 rs35761398 variant; increased NAPE-PDL levels; hyper-expression of CB2 receptor in duodenal mucosa and in the atrophic villous |
| Obesity and NAFLD | Increased risk by FAAH rs324420 polymorphism and CNR2 rs35761398 variant |
| Neuroinflammatory Diseases | Altered expression of CB receptors |
| Type 1 diabetes mellitus | Altered functionality of CB1 receptor in substantia nigra; CB2 receptor down-regulation |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argenziano, M.; Tortora, C.; Bellini, G.; Di Paola, A.; Punzo, F.; Rossi, F. The Endocannabinoid System in Pediatric Inflammatory and Immune Diseases. Int. J. Mol. Sci. 2019, 20, 5875. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20235875
Argenziano M, Tortora C, Bellini G, Di Paola A, Punzo F, Rossi F. The Endocannabinoid System in Pediatric Inflammatory and Immune Diseases. International Journal of Molecular Sciences. 2019; 20(23):5875. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20235875
Chicago/Turabian StyleArgenziano, Maura, Chiara Tortora, Giulia Bellini, Alessandra Di Paola, Francesca Punzo, and Francesca Rossi. 2019. "The Endocannabinoid System in Pediatric Inflammatory and Immune Diseases" International Journal of Molecular Sciences 20, no. 23: 5875. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20235875