Antioxidant Blueberry Anthocyanins Induce Vasodilation via PI3K/Akt Signaling Pathway in High-Glucose-Induced Human Umbilical Vein Endothelial Cells
Abstract
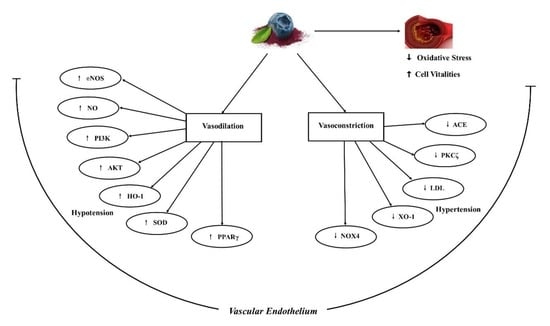
:1. Introduction
2. Results
2.1. Protective Effects of Blueberry Anthocyanins on High-Glucose (HG)-Induced Cytotoxicity in HUVECs
2.2. Protective Effects of Blueberry Anthocyanins against HG-Induced ROS Levels in HUVECs
2.3. Effects of Blueberry Anthocyanins by Upregulating Antioxidant SOD and HO-1 Levels in HG-Induced HUVEC Supernatant
2.4. Vasodilatory Effects of Blueberry Anthocyanins by Upregulating NO and eNOS Levels in HG-Induced HUVEC Supernatant
2.5. Vasodilatory Effects of Blueberry Anthocyanins by Downregulating ACE, XO-1, and LDL Levels in HG-Induced HUVEC Supernatant
2.6. Antioxidant Effects of Blueberry Anthocyanins by Downregulating NOX4 Levels in HG-Induced HUVECs
2.7. Vasodilatory Effects of Blueberry Anthocyanins by Upregulating eNOS and PPARγ Levels in HG-Induced HUVECs
2.8. Vasodilatory Effects of Blueberry Anthocyanins by PI3K/Akt Signaling Pathway and PKC Signaling Pathway in HG-Induced HUVECs
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Antibodies
4.3. Endothelial Cell Culture and Treatment
4.4. Cell Viability Assay
4.5. Reactive Oxygen Species (ROS) Assay
4.6. Enzyme-Linked Immunosorbent Assay (ELISA) Analysis
4.7. Western Blotting
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abreu, D.; Sousa, P.; Matias-Dias, C.; Pinto, F.J. Cardiovascular disease and high blood pressure trend analyses from 2002 to 2016: After the implementation of a salt reduction strategy. BMC Public Health 2018, 18, 722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzone, T.; Chait, A.; Plutzky, J. Cardiovascular disease risk in type 2 diabetes mellitus: Insights from mechanistic studies. Lancet 2008, 371, 1800–1809. [Google Scholar] [CrossRef] [Green Version]
- Egan, B.M.; Li, J.; Hutchison, F.N.; Ferdinand, K.C. Hypertension in the United States 1999-2012: Progress toward healthy people 2020 goals. Circulation 2014, 130, 1692–1699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daiber, A.; Steven, S.; Weber, A.; Shuvaev, V.V.; Muzykantov, V.R.; Laher, I.; Li, H.; Lamas, S.; Münzel, T. Targeting vascular (endothelial) dysfunction. Br. J. Pharmacol. 2017, 174, 1591–1619. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, J.; Nilsson, P.A. A review of risk factors and cardiovascular disease in diabetes care. Eur. J. Cardiovasc. Med. 2011, 3, 21–25. [Google Scholar]
- Förstermann, U.; Xia, N.; Li, H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef]
- Karasu, C. Glycoxidative stress and cardiovascular complications in experimentally-induced diabetes: Effects of antioxidant treatment. Open Cardiovasc. Med. J. 2010, 4, 240–256. [Google Scholar] [CrossRef] [Green Version]
- Rodrigo, R.; Prat, H.; Passalacqua, W.; Araya, J.; Bachler, J.P. Decrease in oxidative stress through supplementation of vitamins C and E is associated with a reduction in blood pressure in patients with essential hypertension. Clin. Sci. 2008, 114, 625–634. [Google Scholar] [CrossRef] [Green Version]
- Landmesser, U.; Harrison, D.G.; Drexler, H. Oxidant stress—A major cause of reduced endothelial nitric oxide availability in cardiovascular disease. Eur. J. Clin. Pharmacol. 2006, 62, 13–19. [Google Scholar] [CrossRef]
- Huang, W.Y.; Davidge, S.T.; Wu, J. Bioactive natural constituents from food sources—Potential use in hypertension prevention and treatment. Crit. Rev. Food Sci. 2013, 53, 615–630. [Google Scholar] [CrossRef]
- Borowska, S.; Brzoska, M.M. Chokeberries (Aronia melanocarpa) and their products as a possible means for the prevention and treatment of noncommunicable diseases and unfavorable health effects due to exposure to xenobiotics. Compr. Rev. Food Sci. Food Saf. 2016, 15, 982–1017. [Google Scholar] [CrossRef] [Green Version]
- Jofré, I.; Pezoa, C.; Cuevas, M.; Scheuermann, E.; Freires, I.A.; Rosalen, P.L.; de Alencar, S.M.; Romero, F. Antioxidant and vasodilator activity of Ugni molinae Turcz. (murtilla) and its modulatory mechanism in hypotensive response. Oxid. Med. Cell. Longev. 2016, 2016, 6513416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [Green Version]
- Miguel, M.G. Anthocyanin: Antioxidant and/or anti-inflammatory activities. J. Appl. Pharm. Sci. 2011, 1, 7–15. [Google Scholar]
- Wu, X.L.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef] [PubMed]
- Różańska, D.; Regulska-Ilow, B. The significance of anthocyanins in the prevention and treatment of type 2 diabetes. Adv. Clin. Exp. Med. 2018, 27, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Hutabarat, R.P.; Xiao, Y.D.; Wu, H.; Wang, J.; Li, D.J.; Huang, W.Y. Identification of anthocyanins and optimization of their extraction from rabbiteye blueberry fruits in Nanjing. J. Food Qual. 2019, 2019, 6806970. [Google Scholar] [CrossRef]
- Huang, W.Y.; Wu, H.; Li, D.J.; Song, J.F.; Xiao, Y.D.; Liu, C.Q.; Zhou, J.Z.; Sui, Z.Q. Protective effects of blueberry anthocyanins against H2O2-induced oxidative injuries in human retinal pigment epithelial cells. J. Agric. Food Chem. 2018, 66, 1638–1648. [Google Scholar] [CrossRef]
- Huang, W.Y.; Yan, Z.; Li, D.J.; Ma, Y.H.; Zhou, J.Z.; Sui, Z.Q. Antioxidant and anti-inflammatory effects of blueberry anthocyanins on high glucose-induced human retinal capillary endothelial cells. Oxid. Med. Cell. Longev. 2018, 2018, 1862462. [Google Scholar] [CrossRef]
- Persson, I.A.; Persson, K.; Andersson, R.G. Effect of Vaccinium myrtillus and its polyphenols on angiotensin-converting enzyme activity in human endothelial cells. J. Agric. Food Chem. 2009, 57, 4626–4629. [Google Scholar] [CrossRef]
- Viskupicova, J.; Blaskovic, D.; Galiniak, S.; Soszyński, M.; Bartosz, G.; Horakova, L.; Sadowska-Bartosz, I. Effect of high glucose concentrations on human erythrocytes in vitro. Redox Biol. 2015, 5, 381–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abraham, N.G.; Kappas, A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol. Rev. 2008, 60, 79–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abreu, I.A.; Cabelli, D.E. Superoxide dismutases-a review of the metal-associated mechanistic variations. BBA-Proteins Proteom. 2010, 1804, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J. Nitric oxide and insulin resistance. Immunoendocrinology 2015, 2, 1–9. [Google Scholar]
- Ooi, S.Y.; Ball, S. ACE inhibitors: Their properties and current role in hypertension. Prescriber 2009, 20, 15–28. [Google Scholar] [CrossRef]
- Feig, D.I. Hyperuricemia and hypertension. Adv. Chronic. Kidney Dis. 2012, 19, 377–385. [Google Scholar] [CrossRef] [Green Version]
- Wadhera, R.K.; Steen, D.L.; Khan, I.; Giugliano, R.P.; Foody, J.M. A review of low-density lipoprotein cholesterol, treatment strategies, and its impact on cardiovascular disease morbidity and mortality. J. Clin. Lipidol. 2016, 10, 472–489. [Google Scholar] [CrossRef] [Green Version]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Kashiwagi, S.; Atochina, D.N.; Lia, Q.; Schleicherb, M.; Ponga, T.; Sessab, W.C.; Huang, P.L. eNOS phosphorylation on serine 1176 affects insulin sensitivity and adiposity. Biochem. Biophys. Res. Commun. 2013, 431, 284–290. [Google Scholar] [CrossRef] [Green Version]
- Kvandova, M.; Barancik, M.; Balis, P.; Puzserova, A.; Majzunova, M.; Dovinova, I. The peroxisome proliferator-activated receptor gamma agonist pioglitazone improves nitric oxide availability, renin-angiotensin system and aberrant redox regulation in the kidney of pre-hypertensive rats. J. Physiol. Pharmacol. 2018, 69, 1–13. [Google Scholar]
- Gao, W.Y.; Dong, X.Y.; Xie, N.; Zhou, C.L.; Fan, Y.H.; Chen, G.Y.; Wang, Y.M.; Wei, T.M.; Zhu, D.L. Dehydroabietic acid isolated from Commiphora opobalsamum causes endothelium-dependent relaxation of pulmonary artery via PI3K/Akt-eNOS signaling pathway. Molecules 2014, 19, 8503–8517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nigro, P.; Abe, J.I.; Woo, C.H.; Satoh, K.; McClain, C.; O’Dell, M.R.; Lee, H.; Lim, J.H.; Li, J.D.; Heo, K.S.; et al. PKCζ decreases eNOS protein stability via inhibitory phosphorylation of ERK5. Blood 2010, 116, 1971–1979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxid. Med. Cell. Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef]
- Li, H.; Horke, S.; Förstermann, U. Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis 2014, 237, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Dhar, I. Oxidative stress as a mechanism of added sugar induced cardiovascular disease. Int. J. Angiol. 2014, 23, 217–226. [Google Scholar]
- Moyer, R.A.; Hummer, K.E.; Finn, C.E.; Frei, B.; Wrolstad, R. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: Vaccinium, rubus, and ribes. J. Agric. Food Chem. 2002, 50, 519–525. [Google Scholar] [CrossRef]
- Jo, Y.N.; Jin, D.E.; Jeong, J.H.; Kim, H.J.; Kim, D.O.; Heo, H.J. Effect of anthocyanins from rabbit-eye blueberry (Vaccinium virgatum) on cognitive function in mice under trimethyltin-induced neurotoxicity. Food Sci. Biotechnol. 2015, 24, 1077–1085. [Google Scholar] [CrossRef]
- Li, X.N.; Liu, H.Y.; Lv, L.Z.; Yan, H.Y.; Yuan, Y. Antioxidant activity of blueberry anthocyanin extracts and their protective effects against acrylamide-induced toxicity in HepG2 cells. Int. J. Food Sci. Technol. 2018, 53, 147–155. [Google Scholar] [CrossRef]
- Huang, W.Y.; Zhang, H.C.; Liu, W.X.; Li, C.Y. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J. Zhejiang Univ. Sci. B 2012, 13, 94–102. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.Y.; Zhu, Y.M.; Li, C.Y.; Sui, Z.Q.; Min, W.H. Effect of blueberry anthocyanins malvidin and glycosides on the antioxidant properties in endothelial cells. Oxid. Med. Cell. Longev. 2016, 2016, 1591803. [Google Scholar] [CrossRef] [Green Version]
- Kahkonen, M.P.; Heinonen, M. Antioxidant activity of anthocyanins and their aglycons. J. Agric. Food Chem. 2003, 51, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Passamonti, S.; Vrhovsek, U.; Mattivi, F. The interaction of anthocyanins with bilitranslocase. Biochem. Biophys. Res. Commun. 2002, 296, 631–636. [Google Scholar] [CrossRef]
- Kattoor, A.J.; Pothineni, N.V.; Palagiri, D.; Mehta, J.L. Oxidative stress in atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Turkseven, S.; Kruger, A.; Mingone, C.J.; Kaminski, P.; Inaba, M.; Rodella, L.F.; Ikehara, S.; Wolin, M.S.; Abraham, N.G. Antioxidant mechanism of heme oxygenase-1 involves an increase in superoxide dismutase and catalase in experimental diabetes. J. Physiol. Heart Circ. Physiol. 2005, 289, H701–H707. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, A.; Kanno, T.; Nishizaki, T. PI3 kinase directly phosphorylates Akt1/2 at Ser473/474 in the insulin signal transduction pathway. J. Endocrinol. 2014, 220, 49–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manning, B.D.; Toker, A. AKT/PKB signaling: Navigating the network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef] [Green Version]
- Vallee, A.; Vallee, J.N.; Lecarpentier, Y. Metabolic reprogramming in atherosclerosis: Opposed interplay between the canonical WNT/beta-catenin pathway and PPAR gamma. J. Mol. Cell Cardiol. 2019, 133, 36–46. [Google Scholar] [CrossRef]
- Sandoo, A.; Veldhuijzen van Zanten, J.J.C.S.; Metsios, G.S.; Carroll, D.; Kitas, G.D. The endothelium and its role in regulating vascular tone. Open Cardiovasc. Med. J. 2010, 4, 302–312. [Google Scholar] [CrossRef]
- Malesev, D.; Kunti, V. Investigation of metal-flavonoid chelates and the determination of flavonoids via metal-flavonoid complexing reactions. J. Serb. Chem. Soc. 2007, 72, 921–939. [Google Scholar] [CrossRef]
- Kuwabara, M. Hyperuricemia, cardiovascular disease, and hypertension. Pulse 2015, 3, 242–252. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, E.A.; Myasoedova, V.A.; Melnichenko, A.A.; Grechko, A.V.; Orekhov, A.N. Small dense low-density lipoprotein as biomarker for atherosclerotic diseases. Oxid. Med. Cell. Longev. 2017, 2017, 1273042. [Google Scholar] [CrossRef] [PubMed]
- Sautin, Y.Y.; Johnson, R.J. Uric acid: The oxidant-antioxidant paradox, Nucleos. Nucleot. Nucl. 2008, 27, 608–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, F.; Fernandes, E.; Roleira, F. Progress towards the discovery of xanthine oxidase inhibitors. Curr. Med. Chem. 2002, 9, 195–217. [Google Scholar] [CrossRef] [PubMed]
- Rouaud, F.; Romero-Perez, M.; Wang, H.; Lobysheva, I.; Ramassamy, B.; Henry, E.; Tauc, P.; Giacchero, D.; Boucher, J.L.; Deprez, E.; et al. Regulation of NADPH-dependent nitric oxide and reactive oxygen species signalling in endothelial and melanoma cells by a photoactive NADPH analogue. Oncotarget 2014, 5, 10650–10664. [Google Scholar] [CrossRef] [Green Version]
- Yousefian, M.; Shakour, N.; Hosseinzadeh, H.; Hayes, A.W.; Hadizadeh, F.; Karimi, G. The natural phenolic compounds as modulators of NADPH oxidases in hypertension. Phytomedicine 2018, 55, 200–213. [Google Scholar] [CrossRef]
- Oubaha, M.; Lin, M.I.; Margaron, Y.; Filion, D.; Price, E.N.; Zon, L.I.; Cote, J.F.; Gratton, J.P. Formation of a PKCζ/β-catenin complex in endothelial cells promotes angiopoietin-1-induced collective directional migration and angiogenic sprouting. Blood 2012, 120, 3371–3381. [Google Scholar] [CrossRef] [Green Version]
- Heo, K.S.; Lee, H.; Nigro, P.; Thomas, T.; Le, N.T.; Chang, E.; McClain, C.; Reinhart-King, C.A.; King, M.R.; Berk, B.C.; et al. PKCζ mediates disturbed flow-induced endothelial apoptosis via p53 SUMOylation. J. Cell Biol. 2011, 193, 867–884. [Google Scholar] [CrossRef]
- Park, H.-J.; Zhang, Y.; Georgescu, S.P.; Johnson, K.L.; Kong, D.; Galper, J.B. Human umbilical vein endothelial cells and human dermal microvascular endothelial cells offer new insights into the relationship between lipid metabolism and angiogenesis. Stem Cell Rev. 2006, 2, 93–102. [Google Scholar] [CrossRef]
- Zhu, C.; Dong, Y.C.; Liu, H.L.; Ren, H.; Cui, Z.H. Hesperetin protects against H2O2-triggered oxidative damage via upregulation of the Keap1-Nrf2/HO-1 signal pathway in ARPE-19 cells. Biomed. Pharmacother. 2017, 88, 124–133. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.; Hutabarat, R.P.; Chai, Z.; Zheng, T.; Zhang, W.; Li, D. Antioxidant Blueberry Anthocyanins Induce Vasodilation via PI3K/Akt Signaling Pathway in High-Glucose-Induced Human Umbilical Vein Endothelial Cells. Int. J. Mol. Sci. 2020, 21, 1575. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21051575
Huang W, Hutabarat RP, Chai Z, Zheng T, Zhang W, Li D. Antioxidant Blueberry Anthocyanins Induce Vasodilation via PI3K/Akt Signaling Pathway in High-Glucose-Induced Human Umbilical Vein Endothelial Cells. International Journal of Molecular Sciences. 2020; 21(5):1575. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21051575
Chicago/Turabian StyleHuang, Wuyang, Ruth Paulina Hutabarat, Zhi Chai, Tiesong Zheng, Weimin Zhang, and Dajing Li. 2020. "Antioxidant Blueberry Anthocyanins Induce Vasodilation via PI3K/Akt Signaling Pathway in High-Glucose-Induced Human Umbilical Vein Endothelial Cells" International Journal of Molecular Sciences 21, no. 5: 1575. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21051575