Figure 1.
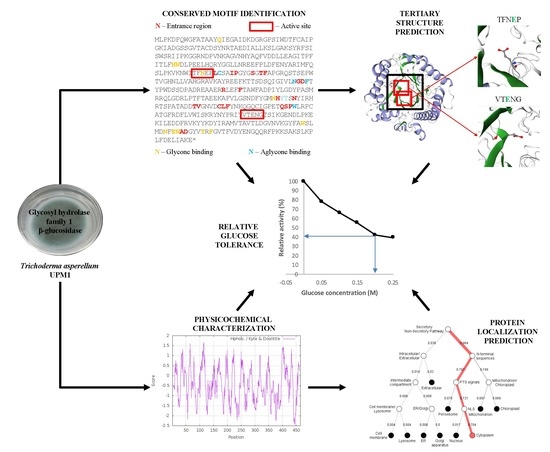
Sequence alignment of TaBgl2 with selected Trichoderma spp. β-glucosidase protein sequences (denoted by their enzyme abbreviations and NCBI/GenBank accession numbers) (TaBgl2, T. asperellum UPM1 Bgl 2 – ARW78142.1; TaBgl, T. asperellum CBS 433.97 glycoside hydrolase family 1 – XP_024766195.1; TgBgl, T. gamsii β-glucosidase – XP_018660766.2; TatBgl, T. atroviride IMI 206040 glycoside hydrolase family 1 – XP_013939543.1; TvBgl, T. virens β-1,4-glucosidase – AJW67427.1; TguBgl, T. guizhouense GH1 β-glucosidase Bgl2 – OPB339337.1; ThBgl, T. harzianum β-glucosidase – KKP02477.1; TrBgl2, T. reesei β-glucosidase – BAA74959.1). Highlighted motifs, in red boxes, include: (A) glycosyl hydrolase family 1 N-terminal signature, with (B) & (C) as catalytic site motifs. Boxed in blue and yellow are suggested glycone and aglycone binding residues, respectively.
Figure 1.
Sequence alignment of TaBgl2 with selected Trichoderma spp. β-glucosidase protein sequences (denoted by their enzyme abbreviations and NCBI/GenBank accession numbers) (TaBgl2, T. asperellum UPM1 Bgl 2 – ARW78142.1; TaBgl, T. asperellum CBS 433.97 glycoside hydrolase family 1 – XP_024766195.1; TgBgl, T. gamsii β-glucosidase – XP_018660766.2; TatBgl, T. atroviride IMI 206040 glycoside hydrolase family 1 – XP_013939543.1; TvBgl, T. virens β-1,4-glucosidase – AJW67427.1; TguBgl, T. guizhouense GH1 β-glucosidase Bgl2 – OPB339337.1; ThBgl, T. harzianum β-glucosidase – KKP02477.1; TrBgl2, T. reesei β-glucosidase – BAA74959.1). Highlighted motifs, in red boxes, include: (A) glycosyl hydrolase family 1 N-terminal signature, with (B) & (C) as catalytic site motifs. Boxed in blue and yellow are suggested glycone and aglycone binding residues, respectively.
Figure 2.
Alignment of TaBgl2 (represented as Chain A) to template Trichoderma reesei Bgl2 associated with Tris (3ahy.1). QMEAN values, displayed by bar height, function to quantify modelling errors and simultaneously estimate the expected model accuracy. α-Helix and β-sheets are colored as blue and green segments, respectively.
Figure 2.
Alignment of TaBgl2 (represented as Chain A) to template Trichoderma reesei Bgl2 associated with Tris (3ahy.1). QMEAN values, displayed by bar height, function to quantify modelling errors and simultaneously estimate the expected model accuracy. α-Helix and β-sheets are colored as blue and green segments, respectively.
Figure 3.
(A) Three-dimensional (3D) structure of TaBgl2 on the side of the active site entrance (boxed). Glutamate residues (E165 and E366) within catalytic motifs (TFNEP and VTENG) are as seen in (B,C), respectively. α-Helix and β-sheets are colored blue and green, respectively.
Figure 3.
(A) Three-dimensional (3D) structure of TaBgl2 on the side of the active site entrance (boxed). Glutamate residues (E165 and E366) within catalytic motifs (TFNEP and VTENG) are as seen in (B,C), respectively. α-Helix and β-sheets are colored blue and green, respectively.
Figure 4.
The subcellular position of predicted TaBgl2 protein presented by (A) SignalP 5.0, (B) Phobius and (C) DeepLoc-1.0 software.
Figure 4.
The subcellular position of predicted TaBgl2 protein presented by (A) SignalP 5.0, (B) Phobius and (C) DeepLoc-1.0 software.
Figure 5.
Alignment of N-terminal amino acid sequences of Humicola grisea Bgl4, Trichoderma reesei BglII and Trichoderma asperellum Bgl2 (TaBgl2). Boxed in blue and yellow are aspartate and lysine residues, respectively.
Figure 5.
Alignment of N-terminal amino acid sequences of Humicola grisea Bgl4, Trichoderma reesei BglII and Trichoderma asperellum Bgl2 (TaBgl2). Boxed in blue and yellow are aspartate and lysine residues, respectively.
Figure 6.
Neighbor-joining phylogram of selected homologous glucose tolerant β-glucosidases and TaBgl2 (ARW78142.1; boxed red) generated via MEGA X. The bootstrap consensus tree is inferred from 1000 replicates, with the confidence values shown next to the branches.
Figure 6.
Neighbor-joining phylogram of selected homologous glucose tolerant β-glucosidases and TaBgl2 (ARW78142.1; boxed red) generated via MEGA X. The bootstrap consensus tree is inferred from 1000 replicates, with the confidence values shown next to the branches.
Figure 7.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) profile of fraction samples (periplasm, cytoplasm and insoluble) for expressed protein visualization from crude protein extracts of host Escherichia coli and transformant TaBgl2 carrying plasmid pET-20b(+)-bgl2. Protein sizes were compared to bands separated from PageRuler™ Unstained Protein Ladder (Lane 1, in all gels) with a ~50 kDa band corresponding to the deduced recombinant TaBgl2 in the E. coli BL21(DE3) transformant (boxed yellow). H, Host; T, Transformant. Biological sample triplicates were prepared for all SDS-PAGE profiles.
Figure 7.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) profile of fraction samples (periplasm, cytoplasm and insoluble) for expressed protein visualization from crude protein extracts of host Escherichia coli and transformant TaBgl2 carrying plasmid pET-20b(+)-bgl2. Protein sizes were compared to bands separated from PageRuler™ Unstained Protein Ladder (Lane 1, in all gels) with a ~50 kDa band corresponding to the deduced recombinant TaBgl2 in the E. coli BL21(DE3) transformant (boxed yellow). H, Host; T, Transformant. Biological sample triplicates were prepared for all SDS-PAGE profiles.
Figure 8.
Specific activity (U/mg) of crude enzyme extracts from the periplasmic and cytoplasmic fractions of Escherichia coli BL21(DE3) host and transformant TaBgl2 carrying plasmid pET-20b(+)-bgl2 with ρ-nitrophenyl-β-d-glucopyranoside (ρNPG) used as a substrate. Biological sample triplicates were prepared for analysis and the specific activities in the periplasm and cytoplasm of host and transformant were shown to be significant (p < 0.05), as per Analysis of Variance (ANOVA) Single Factor analysis.
Figure 8.
Specific activity (U/mg) of crude enzyme extracts from the periplasmic and cytoplasmic fractions of Escherichia coli BL21(DE3) host and transformant TaBgl2 carrying plasmid pET-20b(+)-bgl2 with ρ-nitrophenyl-β-d-glucopyranoside (ρNPG) used as a substrate. Biological sample triplicates were prepared for analysis and the specific activities in the periplasm and cytoplasm of host and transformant were shown to be significant (p < 0.05), as per Analysis of Variance (ANOVA) Single Factor analysis.
Figure 9.
Glucose tolerance of recombinant TaBgl2 against glucose concentrations up to 0.25 M, expressed in relative activity (%). Biological sample triplicates were prepared for analysis.
Figure 9.
Glucose tolerance of recombinant TaBgl2 against glucose concentrations up to 0.25 M, expressed in relative activity (%). Biological sample triplicates were prepared for analysis.
Table 1.
Prediction of secondary structures within β-glucosidases of selected Trichoderma spp., using ExPASY’s GOR IV.
Table 1.
Prediction of secondary structures within β-glucosidases of selected Trichoderma spp., using ExPASY’s GOR IV.
| Description | Accession Number | GOR IV Analyses |
|---|
| α-Helix (Hh) (%) | Extended Strand (Ee) (%) | Random Coils (%) |
|---|
| β-Glucosidase 2 (Trichoderma asperellum) * | ARW78142.1 | 26.45 | 18.92 | 54.62 |
| Glycoside hydrolase family 1 protein (Trichoderma asperellum CBS 433.97) | XP_024766195.1 | 25.38 | 18.92 | 55.70 |
| β-Glucosidase (Trichoderma gamsii) | XP_018660766.2 | 27.31 | 18.28 | 54.41 |
| Glycoside hydrolase family 1 protein (Trichoderma atroviride IMI 206040) | XP_013939543.1 | 25.81 | 19.35 | 54.84 |
| β-1,4-Glucosidase (Trichoderma virens) | AJW67427.1 | 24.84 | 19.34 | 55.82 |
| GH1 β-glucosidase BGL2/CEL1a (Trichoderma guizhouense) | OPB39337.1 | 24.30 | 17.85 | 57.85 |
| β-Glucosidase (Trichoderma harzianum) | KKP02477.1 | 24.52 | 17.63 | 57.85 |
| β-Glucosidase (Trichoderma reesei) | BAA74959.1 | 24.03 | 18.03 | 57.94 |
Table 2.
Physicochemical parameters of β-glucosidases from selected Trichoderma spp., computed using ExPASY’s ProtParam tool.
Table 2.
Physicochemical parameters of β-glucosidases from selected Trichoderma spp., computed using ExPASY’s ProtParam tool.
| Description | Accession Number | pI | R+ | R− | EC (M−1·cm−1) | II | Stability | AI | GRAVY | Formula | TNA |
|---|
| β-Glucosidase 2 (Trichoderma asperellum) * | ARW78142.1 | 5.55 | 60 | 52 | 102,135 | 31.25 | Stable | 69.27 | −0.450 | C2395H3581N631O700S12 | 7319 |
| Glycoside hydrolase family 1 protein (Trichoderma asperellum CBS 433.97) | XP_024766195.1 | 5.45 | 60 | 51 | 102,135 | 31.25 | Stable | 69.27 | −0.449 | C2393H3575N631O701S12 | 7312 |
| β-Glucosidase (Trichoderma gamsii) | XP_018660766.2 | 5.23 | 63 | 51 | 103,625 | 32.33 | Stable | 68.65 | −0.485 | C2397H3576N630O706S12 | 7321 |
| Glycoside hydrolase family 1 protein (Trichoderma atroviride IMI 206040) | XP_013939543.1 | 5.30 | 62 | 51 | 103,625 | 31.58 | Stable | 69.89 | −0.464 | C2401H3587N631O705S12 | 7336 |
| β-1,4-Glucosidase (Trichoderma virens) | AJW67427.1 | 5.53 | 57 | 49 | 102,135 | 31.80 | Stable | 68.86 | −0.442 | C2338H3489N619O685S12 | 7143 |
| GH1 β-glucosidase BGL2/CEL1a (Trichoderma guizhouense) | OPB39337.1 | 5.10 | 64 | 50 | 104,990 | 25.55 | Stable | 72.19 | −0.450 | C2409H3589N627O704S11 | 7340 |
| β-Glucosidase (Trichoderma harzianum) | KKP02477.1 | 5.11 | 64 | 50 | 104,990 | 26.23 | Stable | 71.35 | −0.454 | C2409H3589N627O704S12 | 7341 |
| β-Glucosidase (Trichoderma reesei) | BAA74959.1 | 5.33 | 60 | 49 | 102,010 | 27.54 | Stable | 70.60 | −0.396 | C2368H3534N628O693S11 | 7234 |
Table 3.
Amino acid composition (%) of selected Trichoderma spp. β-glucosidases computed using ExPASy’s ProtParam tool.
Table 3.
Amino acid composition (%) of selected Trichoderma spp. β-glucosidases computed using ExPASy’s ProtParam tool.
| Amino Acid | ARW78142.1 * | XP_024766195.1 | XP_018660766.2 | XP_013939543.1 | AJW67427.1 | OPB39337.1 | KKP02477.1 | BAA74959.1 |
|---|
| Ala (A) | 7.7 | 7.7 | 7.5 | 7.5 | 7.7 | 7.3 | 7.3 | 9.0 |
| Arg (R) | 5.8 | 5.8 | 5.8 | 5.8 | 5.9 | 5.6 | 5.6 | 5.8 |
| Asn (N) | 4.5 | 4.7 | 4.5 | 4.7 | 4.8 | 4.7 | 4.7 | 4.1 |
| Asp (D) | 7.3 | 7.3 | 7.7 | 7.5 | 7.3 | 8.2 | 8.0 | 7.9 |
| Cys (C) | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 | 1.1 | 1.1 | 1.1 |
| Gln (Q) | 2.8 | 2.8 | 2.8 | 2.8 | 2.9 | 2.6 | 2.6 | 2.6 |
| Glu (E) | 5.6 | 5.6 | 5.8 | 5.8 | 5.3 | 5.6 | 5.8 | 4.9 |
| Gly (G) | 8.0 | 8.0 | 8.0 | 7.7 | 8.1 | 8.2 | 8.2 | 9.0 |
| His (H) | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.7 |
| Ile (I) | 5.4 | 5.4 | 5.8 | 5.6 | 5.5 | 5.2 | 5.2 | 5.2 |
| Leu (L) | 6.9 | 6.9 | 6.7 | 6.9 | 6.6 | 8.0 | 7.7 | 6.7 |
| Lys (K) | 5.4 | 5.2 | 5.2 | 5.2 | 4.8 | 5.2 | 5.2 | 4.7 |
| Met (M) | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 | 1.5 | 1.3 |
| Phe (F) | 6.5 | 6.5 | 6.2 | 6.2 | 6.4 | 6.2 | 6.2 | 6.0 |
| Pro (P) | 5.6 | 5.6 | 5.8 | 5.6 | 5.5 | 5.8 | 5.8 | 6.0 |
| Ser (S) | 6.0 | 6.0 | 5.8 | 5.6 | 6.2 | 5.2 | 5.2 | 5.8 |
| Thr (T) | 6.0 | 6.0 | 6.0 | 6.2 | 6.2 | 5.6 | 5.6 | 5.2 |
| Trp (W) | 2.6 | 2.6 | 2.6 | 2.6 | 2.6 | 2.6 | 2.6 | 2.6 |
| Tyr (Y) | 5.2 | 5.2 | 5.4 | 5.4 | 5.3 | 5.6 | 5.6 | 5.2 |
| Val (V) | 4.7 | 4.7 | 4.3 | 4.7 | 4.8 | 4.7 | 4.7 | 5.4 |
Table 4.
Comparison of hydrophobic scores and positions of selected Trichoderma spp. β-glucosidases using ExPASY ProtScale tool.
Table 4.
Comparison of hydrophobic scores and positions of selected Trichoderma spp. β-glucosidases using ExPASY ProtScale tool.
| Description | Accession Number | Position | Score |
|---|
| Min | Max | Min | Max |
|---|
| β-Glucosidase 2 (Trichoderma asperellum) * | ARW78142.1 | 445 | 114 | −2.444 | 1.667 |
| Glycoside hydrolase family 1 protein (Trichoderma asperellum CBS 433.97) | XP_024766195.1 | 445 | 114 | −2.444 | 1.667 |
| β-Glucosidase (Trichoderma gamsii) | XP_018660766.2 | 445 | 114 | −2.444 | 1.667 |
| Glycoside hydrolase family 1 protein (Trichoderma atroviride IMI 206040) | XP_013939543.1 | 445 | 114 | −2.444 | 1.667 |
| β-1,4-Glucosidase (Trichoderma virens) | AJW67427.1 | 445 | 114 | −2.444 | 1.667 |
| GH1 β-glucosidase BGL2/CEL1a (Trichoderma guizhouense) | OPB39337.1 | 445 | 195 | −2.444 | 1.689 |
| β-Glucosidase (Trichoderma harzianum) | KKP02477.1 | 445 | 195 | −2.444 | 1.689 |
| β-Glucosidase (Trichoderma reesei) | BAA74959.1 | 446 | 114 | −2.489 | 1.667 |
Table 5.
Codon usage of Escherichia coli corresponding to each amino acid and ‘stop’ signal (abbreviated), compared to codons present in native (bgl2n) and codon optimized (bgl2co) TaBgl2 gene sequences.
Table 5.
Codon usage of Escherichia coli corresponding to each amino acid and ‘stop’ signal (abbreviated), compared to codons present in native (bgl2n) and codon optimized (bgl2co) TaBgl2 gene sequences.
| Amino Acid | Codon | Frequency | Amino Acid | Codon | Frequency |
|---|
| E. coli | bgl2n | bgl2co | E. coli | bgl2n | bgl2co |
|---|
| ILE | ATT | 0.49 | 0.28 | 0.56 | TRP | TGG | 1.00 | 1.00 | 1.00 |
| | ATC | 0.39 | 0.72 | 0.44 | CYS | TGT | 0.46 | 0.17 | 0.00 |
| | ATA | 0.11 | 0.00 | 0.00 | | TGC | 0.54 | 0.83 | 1.00 |
| LEU | CTT | 0.12 | 0.16 | 0.00 | ALA | GCT | 0.18 | 0.17 | 0.00 |
| | CTC | 0.10 | 0.16 | 0.00 | | GCC | 0.26 | 0.61 | 0.00 |
| | CTA | 0.04 | 0.00 | 0.00 | | GCA | 0.23 | 0.06 | 0.00 |
| | CTG | 0.47 | 0.50 | 1.00 | | GCG | 0.33 | 0.17 | 1.00 |
| | TTA | 0.14 | 0.00 | 0.00 | GLY | GGT | 0.35 | 0.14 | 0.59 |
| | TTG | 0.13 | 0.19 | 0.00 | | GGC | 0.37 | 0.57 | 0.41 |
| VAL | GTT | 0.28 | 0.27 | 0.50 | | GGA | 0.13 | 0.24 | 0.00 |
| | GTC | 0.20 | 0.41 | 0.00 | | GGG | 0.15 | 0.05 | 0.00 |
| | GTA | 0.17 | 0.05 | 0.00 | PRO | CCT | 0.18 | 0.15 | 0.00 |
| | GTG | 0.35 | 0.27 | 0.50 | | CCC | 0.13 | 0.50 | 0.00 |
| | TTT | 0.58 | 0.47 | 0.47 | | CCA | 0.20 | 0.23 | 0.00 |
| | TTC | 0.42 | 0.53 | 0.53 | | CCG | 0.49 | 0.12 | 1.00 |
| MET | ATG | 1.00 | 1.00 | 1.00 | THR | ACT | 0.19 | 0.18 | 0.00 |
| STOP | TAA | 0.61 | 1.00 | 1.00 | | ACC | 0.40 | 0.36 | 1.00 |
| | TGA | 0.30 | 0.00 | 0.00 | | ACA | 0.17 | 0.11 | 0.00 |
| | TAG | 0.09 | 0.00 | 0.00 | | ACG | 0.25 | 0.36 | 0.00 |
| PHE | TTT | 0.58 | 0.47 | 0.47 | TYR | TAT | 0.59 | 0.33 | 0.46 |
| | TTC | 0.42 | 0.53 | 0.53 | | TAC | 0.41 | 0.67 | 0.54 |
| SER | TCT | 0.17 | 0.21 | 0.00 | ARG | CGT | 0.36 | 0.04 | 1.00 |
| | TCC | 0.15 | 0.21 | 0.00 | | CGC | 0.36 | 0.52 | 0.00 |
| | TCA | 0.14 | 0.07 | 0.00 | | CGA | 0.07 | 0.22 | 0.00 |
| | TCG | 0.14 | 0.21 | 0.00 | | CGG | 0.11 | 0.04 | 0.00 |
| | AGT | 0.16 | 0.04 | 0.00 | | AGA | 0.07 | 0.15 | 0.00 |
| | AGC | 0.25 | 0.25 | 1.00 | | AGG | 0.04 | 0.04 | 0.00 |
| GLN | CAA | 0.34 | 0.15 | 0.31 | ASP | GAT | 0.63 | 0.44 | 0.47 |
| | CAG | 0.66 | 0.85 | 0.69 | | GAC | 0.37 | 0.56 | 0.53 |
| ASN | AAT | 0.49 | 0.14 | 0.00 | LYS | AAA | 0.74 | 0.24 | 0.40 |
| | AAC | 0.51 | 0.86 | 1.00 | | AAG | 0.26 | 0.76 | 0.60 |
| HIS | CAT | 0.57 | 0.14 | 0.00 | GLU | GAA | 0.68 | 0.27 | 0.42 |
| | CAC | 0.43 | 0.86 | 1.00 | | GAG | 0.32 | 0.73 | 0.58 |
Table 6.
Degenerate primers from the conserved regions of β-glucosidase amino acid sequence variants from Trichoderma sp. belonging to the Bgl2/Cel1A family.
Table 6.
Degenerate primers from the conserved regions of β-glucosidase amino acid sequence variants from Trichoderma sp. belonging to the Bgl2/Cel1A family.
| Primer | Conserved Region | Sequence (5′ to 3′) |
|---|
| DGFAMILYF | YQIEGA | TAYCARATHGARGGNGC |
| DGFAMILYR | DFYGMN | TTCATNCCRTARAARTC |
Table 7.
Gene specific primers designed for rapid amplification of cDNA ends (RACE) PCR. 5′-GATTACGCCAAGCTT-3′ sequence introduced and highlighted in bold, is for cloning into vector, as suggested by SMARTer ® RACE 5′/3′ Kit User Manual.
Table 7.
Gene specific primers designed for rapid amplification of cDNA ends (RACE) PCR. 5′-GATTACGCCAAGCTT-3′ sequence introduced and highlighted in bold, is for cloning into vector, as suggested by SMARTer ® RACE 5′/3′ Kit User Manual.
| Primer | Sequence (5′ to 3′) |
|---|
| GSPFAM1F | GATTACGCCAAGCTTGACGACCTGCTGGAAGCGGGCATCACC |
| GSPFAM1R | GATTACGCCAAGCTTATCGCTGGTGGTCTTGAACTCCTCTCG |