1. Introduction
Implants have been used to improve loss of function, replace lost tissue, or optimize appearance. Various medical devices with different raw materials and shapes have been developed. Hydroxyapatite (HAp) is the principal inorganic constituent of hard tissues (bone and teeth) and has been widely applied in tissue engineering as an orthopedic and dental material due to its biocompatibility and osteoconductivity. Furthermore, HAp can form very tight bonds with living bone, resulting in the promotion of new bone formation. In many researches, HAp has been employed as bioactive coatings onto titanium [
1] and polymer substrates [
2] for enhanced skeletal fixation. Calcium phosphates, mainly as HAp, have been used in the field of bone tissue engineering, though the low bioresorbability of sintered HAp resulted in the cause of implant-related infection. On the other hand, HAp is also widely used as a column material in affinity chromatography for the separation of various proteins because of its ability to absorb proteins, amino acids and other substances [
3,
4,
5]. This means that bacteria can also prefer to adhere to the HAp surface and form biofilms. To avoid the incidence of implant-related infections, several approaches have been employed [
6,
7,
8]. In particular, surface modifications with antibacterial coatings, immobilized molecules, or light-activated molecules are widely explored novel strategies [
9]. While antibiotics are still essential for the success of surgical procedures, the increase in bacterial resistance to conventional antibiotics has necessitated the development of alternative therapies.
Causative pathogens are reported to be
Staphylococcus aureus, coagulase-negative
staphylococci,
Enterococcus spp., and
Escherichia coli in surgical site infections (SSIs), which were defined as infections by organisms occurring up to 30 days after surgery [
10,
11]. There is a need to find alternatives with a broad spectrum of activity against several species of bacteria.
Antimicrobial peptides and proteins (AMPs) have attracted much attention as a promising alternative to antibiotics. AMPs are the host defense peptides and are key elements of innate immunity [
12]. Targeting of the bacterial membrane and the concomitant loss of membrane integrity is a well-accepted mechanism of action of AMPs [
13,
14]. In addition, several reports demonstrated that AMPs can disturb a series of cellular processes and metabolic functions [
15]. AMP’s multiple modes of action would be superior to antibiotics, which act only on one specific target. These modes of action enable reducing the bacterial drug resistance [
16].
Protamine, which is an arginine-rich protein extracted from fish milt, is a promising potential AMP. Protamine is a cationic AMP that inhibits or kills many microorganisms, including bacteria and fungi. The electrostatic interaction between (positively charged) protamine and (negatively charged) bacterial membrane is suggested to be the driving force for the expression of the protamine bactericidal activity. However, the precise mechanism of action of protamine at the molecular level remains unclear. Additionally, protamine, biomedical application, has been focused in the field of tissue engineering and regenerative medicine. Basic protamine molecules complexed with acidic molecules such as heparin, flagmin, and DNA form complexes via ionic interaction. Nano/microparticles were generated by electrostatic interaction and can function as immobilizers or attractants of various cytokines. Those nano/microparticles have been applied as biomaterials such as cell carriers, protein carriers, and growth factor carriers [
17,
18].
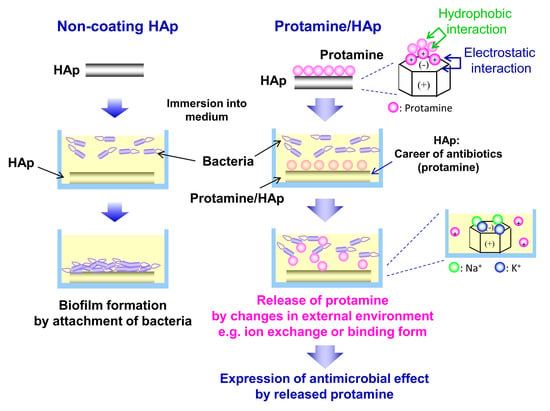
In this study, to develop a new bone substitute with antimicrobial activity, protamine was applied to biomaterials. We evaluated the bactericidal properties of protamine-loaded HAp (protamine/HAp) and demonstrated its suitability for use in bone tissue engineering.
3. Discussion
As previous antibiofilm therapeutics have been unsatisfactory, current research has focused on preventive strategies and, particularly, on implant surface modifications. Several strategies, namely the antiadhesive, contact-active, and biocide release strategies, can be pursued to obtain antimicrobial coatings by chemical modifications [
9]. Among these, we focused on AMPs [
13,
21] and fabricated the novel protamine-loaded HAp biomaterial that inhibits biofilm formation. HAp is most frequently used as a bone-substitute material in orthopedic and dental fields because of its similarity to bone in chemical composition and its direct bonding to bone.
The levels of the adsorption of protamine to HAp powders increased with increasing protamine concentrations in a dose-dependent manner and essentially followed the Langmuir adsorption isotherm. The zeta potential of the protamine/HAp powders also increased with increasing protamine concentrations. This meant that adsorption occurred because of the electrostatic interaction between the amine groups of arginine and the hydroxyl and/or phosphate groups of HAp. In this study, isotropic HAp was used; however, the negatively charged c-planes of HAp can be expected to adsorb large amounts of protamine [
22]. Furthermore, HAp with a fine structure and a large specific surface area will be effective for increasing the loading amount of protamine.
Next, to investigate the interaction between protamine/HAp and bacteria, viability tests using protamine/HAp discs were performed (
Figure 3).
Escherichia coli adhered and formed biofilms on HAp, although the number of living bacteria decreased. Interestingly, the formation of large aggregates was observed for high dosages of protamine/HAp discs (over 1000 μg·mL
−1). For the intermediate levels of protamine (500–1000 μg·mL
−1), we observed elongated
E. coli that could not divide (
Figure 4). This phenomenon was also observed in planktonic bacteria (
Figure S1). Bacterial elongation, called filamentation, was also obtained by treatment with indolicidin [
23] that can permeabilize the bacterial membrane and bind to DNA, resulting in the inhibition of DNA synthesis. Protamine can also bind to DNA and form DNA/protamine complex by electrostatic forces, which was applied to gene delivery [
17]. The interaction of protamine and DNA also prevents DNA synthesis [
24,
25].
As for the release profile of protamine, the amount of the released protamine increased with the increase in the adsorbed protamine concentration (
Figure 5). Protamine adsorbs HAp through electrostatic and/or hydrophobic interactions. HAp has an ion-exchange ability for the exchange of cations with the calcium ion sites and the exchange of anions with the phosphate groups or hydroxide ion sites. In fact, changes in the salt concentration in the LB medium affected the desorption of protamine. These data imply that the release of protamine from HAp can be controlled by adjusting the salt concentration in the surrounding environment. Furthermore, a previous investigation reported that proteins can adsorb in a multilayer fashion on the HAp surface. The increase in the distance due to the multilayers weakened the electrostatic interaction between the protein molecules and HAp [
26]. Thus, we observed the easy release from protamine/HAp (over 1000 μg·mL
−1) that was higher than the saturated adsorption level. This phenomenon was also observed in our previous study [
27]. These results indicate that bacteria were attacked by both immobilized and released protamine. The use of protamine/HAp(500) would be a good candidate for an antibacterial bone substitute, because released protamine from protamine/HAp(500) could damage the bacterial membrane and lead to cell death. HAp enables to release protamine by ion-exchange for the long term. In addition, it is expected that using bioresorbable materials such as tricalcium phosphate (TCP) make it possible to release protamine simultaneously with the resorption of materials by osteoclasts.
As previously reported [
13,
14,
28], AMPs, including protamine, kill bacteria by acting on bacterial membranes or cell walls. Generally, AMP is amphiphilic and positively charged and has both hydrophobic and hydrophilic parts [
12,
16]. Positively charged cationic peptides can interact with negatively charged cell membranes via electrostatic interactions and adsorb the membrane. After binding to the membrane, AMPs can form pores and disintegrate the membrane bilayer structure, leading to bacterial death. Several models of membrane permeation by peptides were provided, such as barrel-stave pore, toroidal pore, carpet model, and detergent model [
21]. In this study, to investigate the interaction between protamine and the bacteria, the zeta potential of the bacterial cell membrane was measured. Our data showed that protamine exhibited antibacterial activity in a concentration-dependent manner against both Gram-negative and Gram-positive bacteria (
Figure 6 and
S2). Additionally, the surface potential of the bacteria was positively shifted by the treatment with protamine. These results agree with the results of previous studies [
27,
29]. The positively charged AMPs interact with the negatively charged cell membranes through electrostatic interactions and undergo membrane adsorption and conformational changes. LIVE/DEAD staining also showed that the bacteria adhered on the protamine/HAp disc (over 500 μg·mL
−1) were dead due to the disruption of the membrane by the attached protamine. Additionally, we observed the differences of susceptibility to protamine between
E. coli and
S. aureus, which might be caused by the differences of the bacterial membrane structures. In the present study, S. aureus were more susceptible to protamine, and a low concentration of protamine (100 μg·mL
−1) could disrupt the membrane, leading to cell death. In contrast to
S. aureus, protamine would be able to past the cell wall without causing damage and bind to DNA in
E. coli [
30]. The inhibition of the DNA synthesis of
E. coli changed their morphology without achieving cell division. However, the precise mechanism of action of protamine remains unknown, and further studies are needed to clarify other mechanisms.
With regards to the biocompatibility of AMPs, it was previously found that AMPs can selectively bind to the outer surface of the negatively charged bacterial cell membranes without interacting with the outer surface of the neutral eukaryotic cell membranes [
13]. In our study, a high dosage of released protamine exhibited a cytotoxic effect. In contrast, the in vivo assessment also showed that protamine/HAp can directly bond to bone (
Figure 8). There are some differences, such as protamine concentration, cell numbers, and cell types, between in vitro and in vivo situations. The discrepancy between the in vitro and in vivo biocompatibility of protamine resulted from blood circulation. Protamine has already been clinically applied as a heparin antagonist and, therefore, is known to be safe [
31]. In medical use, protamine sulfate is normally administered at the dose of 10–15 mg·mL
−1 [
32]. Therefore, the concentration of protamine in this study would be biocompatible. However, since pathogenic microbes become peptide-resistant after long-term use, the side effects of long-term use should be investigated. Taken together, our results show that protamine-loaded medical devices can inhibit biofilm formation and reduce the incidence of implant-associated infections.
4. Materials and Methods
4.1. Preparation and Characterization of Protamine-HAp Powders and Discs
Various concentrations of protamine sulfate solutions (125–2000 μg·mL−1) were diluted by phosphate-buffered saline (PBS). HAp powder (1.5 g; HAp-100; Taihei Chemical Inc., Osaka, Japan) were added into each protamine solution (45 mL) and incubated for 48 h at room temperature. The samples were centrifuged for 15 min at 8.000 rpm, and the supernatant was assayed for the protein using the Bio-Rad (Hercules, CA, USA) protein assay following the manufacturer’s instructions. Powders were washed in PBS 5 times and freeze-dried. To fabricate the discs (diameter: 15 mm, thickness: 1–2 mm), the resulting powders (0.30 g) were uniaxially compressed at 50 MPa.
Measurements of zeta potentials of protamine/HAp powders were carried out using a laser-Doppler velocimeter (ELS-6000; Otsuka Electronics, Osaka, Japan) in a 10 mM NaCl solution at room temperature. The zeta potential was calculated from the measured electrophoretic mobility. The complete experiment was repeated for a total of three separate assays. The morphological observation of the sample powders was performed at an accelerating voltage of 15 kV by scanning electron microscopy (SEM; JSM-6390LA; JEOL, Tokyo, Japan).
4.2. Antimicrobial Evaluation
To evaluate the antibacterial activity, Escherichia coli (E. coli, ATCC 27325) and Staphylococcus aureus (S. aureus, ATCC 6538) were used in this study. Bacteria were grown in a culture broth (nutrient broth from LB; Wako, Osaka, Japan) and culture agar (nutrient agar from LB; Wako, Osaka, Japan) at 37 °C in an incubator.
4.2.1. Antimicrobial Susceptibility Tests
Using the microtiter broth dilution method [
19], the antimicrobial susceptibility tests of protamine against
E. coli and
S. aureus were performed. In brief, protamine (Maruhanichiro Inc., Tokyo, Japan) was solubilized in PBS to a final concentration of 100 mg∙mL
−1 and sterilized by filter with a 0.22-μm pore size. Protamine solutions then were diluted to yield final concentrations of 0.5, 1.0, 2.0, and 4.0 mg mL
−1. Dilutions were dispensed (0.02 mL/well) in the wells of a polypropylene microtiter plate already containing 0.16 mL/well of the prepared 1 × 10
6 cfu mL
−1 bacteria inoculum and 0.02 mL/well of Alamar blue (Invitrogen, Carlsbad, CA, USA). Final protamine concentrations of 50, 100, 200, and 400 μg∙mL
−1 were tested. Each concentration was tested in triplicate. Samples were incubated for 18 h at 37 °C without shaking. The bacterial growth in suspension was monitored at 570 nm and 600 nm. The relative cell viability (%) was calculated by a ratio of optical density (OD)
sample/OD
control × 100 for each of the values. The experiment was repeated for a total of three separate assays.
4.2.2. Measurement of the Surface Charge of Bacteria
The zeta potential analyses were carried out using an ELSZ-1000 (Otsuka Electronics, Osaka, Japan) at room temperature. Protamine solutions were prepared at the concentrations ranging from 50 to 400 μg·mL−1 using 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer solution (pH 7.4), containing 150 mM NaCl. Protamine stock dilution (0.1 mL) was mixed with 0.9 mL of the bacterial suspension. The bacterial suspensions were dispensed into microtubes and incubated for 15 min at room temperature. The zeta potential was calculated from the electrophoretic mobility. The complete experiment was repeated for a total of three separate assays.
4.2.3. LIVE/DEAD Staining
Bacteria cultured on various concentrations of protamine/HAp discs were examined for cell viability using a BacLight LIVE/DEAD staining kit (Invitrogen, Carlsbad, CA, USA). Equal volumes of SYTO® 9 and propidium iodide (PI) were mixed in microtube and then the dye mixture solution was added to PBS. Following resuspension of each sample in 1 mL of the staining solution, samples (bacteria on the discs or in the supernatants) were incubated for 15 min in the dark at room temperature. After washing with PBS, samples then were imaged using fluorescence microscopy (IX71; Olympus, Tokyo, Japan). Each concentration was tested in triplicate.
4.2.4. Measurement of the Cell Concentration by Optical Density
Optical density (OD), measured in a spectrophotometer (Gene Quant 100; GE Healthcare, Piscataway, NJ, USA), can be used as a measure of the concentration of bacteria in a suspension. Bacterial concentrations were determined by measuring the OD at 600 nm. The OD values of the inoculum were standardized to OD = 1.0 at 600 nm (approximately 8 × 108 CFU·mL−1).
4.3. Release Profiles of Protamine from Protamine/HAp Discs
The protamine/HAp discs with different protamine contents were placed on 24-well plates (BD, Franklin Lake, NJ, USA), which were filled with bacterial suspension at 37 °C. After 24 h culture, concentrations of protamine released from discs were then measured using the Bio-Rad protein assay.
4.4. Biocompatibility Evaluation
4.4.1. Evaluation of Biocompatibility In Vitro
Human osteoblasts, MG-63 cells, were obtained from ATCC
® CRL-1427
TM (Manassas, VA, USA). MG-63 cells were cultured in Eagle’s minimum essential medium (MEM; Sigma, St. Louis, MO, USA) supplemented with 10% heat-inactivated fetal bovine serum (Sigma, St. Louis, MO, USA) and 0.1% antibiotics (penicillin-streptomycin solution, Sigma) in a humidified atmosphere containing 5% CO
2 at 37 °C. According to ISO 10993-5:2009 “Tests for Cytotoxicity—In vitro Methods” [
20], extracts were obtained in a separate culture medium (0.1 g·mL
−1 of the culture medium for 24 h at 37 °C). For cytotoxicity test, cells were seeded at 5 × 10
3 cells·well
–1 on 96-well plates and cultured for 24 h at 37 °C/5% CO
2. Each extract (0.1 mL) was then applied to cultured cells. After 24 h and 72h of incubation, culture medium was replaced with a fresh medium. Subsequently, 5 mg·mL
−1 of MTT solution (10 μL) was added, and cells were cultured for 4 h at 37 °C/5% CO
2. The MTT solution was then removed, and formed formazan crystals were solubilized by DMSO (100 μL) per well. After that, the absorbances at 570 nm (measurement wavelength) and 650 nm (reference wavelength) of each well were quantified by a microplate reader (Multiskan FC; Thermo Fisher Scientific, Waltham, MA, USA). The relative cell viability (%) was calculated by a ratio of OD
sample/OD
control (HAp at 24 h) × 100 for each of the values.
4.4.2. Evaluation of Biocompatibility In Vivo
To assess the biocompatibility and osteoconductivity of protamine/HAp discs in vivo, 16-week-old male rabbits (average weight: 3 kg) were used in this study. The specimens (diameter: 4 mm, height: 8 mm) were sterilized by ethylene oxide gas. The tibia of a rabbit was exposed, and cylindrical defects (4.2 mm in diameter) were drilled in the epiphysis of the tibia. The HAp or protamine/HAp discs were then implanted into the defect for 8 weeks. After implantation, the rabbit was sacrificed using sodium pentobarbital, and the tibia was removed. For undecalcified histological analysis, harvested implants were fixed 70% ethanol, dehydrated in an alcohol series, defatted, embedded in methylmethacrylate, and cut into 7 μm sections using a microtome. After staining with Villanueva bone stain, these specimens were observed under light microscopy (IX71; Olympus, Tokyo, Japan). All experiments were approved by the Keio University Institutional Animal Care and Use Committee (09067-(10)).