Mutation in Sodium-Glucose Cotransporter 2 Results in Down-Regulation of Amyloid Beta (A4) Precursor-Like Protein 1 in Young Age, Which May Lead to Poor Memory Retention in Old Age
Abstract
:1. Introduction
2. Results
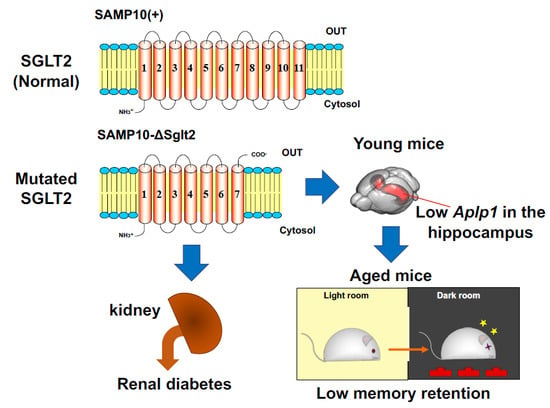
2.1. Characteristics of SAMP10-ΔSglt2, SAMP10(+) and SAMR1
2.2. Memory Retention and Depression-Like Behavior
2.3. Transcriptome and the Levels of Gene Expression
2.4. Effect of Sglt2 Mutation on Gene Expression in Hippocampus
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Measurements of Physiological Parameters, Glucose Levels, and Brain Weight
4.3. Measurements of Behavioral Task
4.4. Measurement of DNA Microarray and Principal Component Analyses
4.5. Quantitative Real-Time Reverse Transcription PCR (qRT-PCR)
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SAMP10 | Senescence-accelerated mouse prone 10 |
| SAMP10-ΔSglt2 | SAMP10/TaSlc-Slc5a2slc, SAMP10 with mutation in SGLT2 |
| SAMP10(+) | SAMP10/TaIdrSlc, SAMP10 without mutation |
| SAMR1 | SAMR1/TaSlc, senescence-resistant strain |
| SGLT2 | sodium-glucose cotransporter 2 |
| Aplp1 | amyloid beta (A4) precursor-like protein 1 |
| Camkv | CaM kinase-like vesicle-associated |
| Cyr61 | Cysteine rich protein 61 |
| PSD95 | Postsynaptic density 95 |
| Syn | Synaptophysin |
References
- Takeda, T.; Hosokawa, M.; Higuchi, K. Senescence-accelerated mouse (SAM). In The Senescence-Accelerated Mouse (SAM): Achievement and Future Directions; Takada, T., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 3–14. [Google Scholar]
- Takeda, T.; Hosokawa, M.; Takeshita, S.; Irino, M.; Higuchi, K.; Matsushita, T.; Tomita, Y.; Yasuhira, K.; Hamamoto, H.; Shimizu, K.; et al. A new murine model of accelerated senescence. Mech. Ageing Dev. 1981, 17, 183–194. [Google Scholar] [CrossRef]
- Shimada, A.; Ohta, A.; Akiguchi, I.; Takeda, T. Inbred SAM-P/10 as a mouse model of spontaneous, inherited brain atrophy. J. Neuropathol. Exp. Neurol. 1992, 51, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Shimada, A.; Ohta, A.; Akiguchi, I.; Takeda, T. Age-related deterioration in conditional avoidance task in the SAM-P/10 mouse, an animal model of spontaneous brain atrophy. Brain Res. 1993, 608, 266–272. [Google Scholar] [CrossRef]
- Takeda, T. Senescence-accelerated mouse (SAM) with special references to neurodegeneration models, SAMP8 and SAMP10 mice. Neurochem. Res. 2009, 34, 639–659. [Google Scholar] [CrossRef]
- Del Valle, J.; Duran-Vilaregut, J.; Manich, G.; Casadesús, G.; Smith, M.A.; Camins, A.; Pallàs, M.; Pelegrí, C.; Vilaplana, J. Early amyloid accumulation in the hippocampus of SAMP8 mice. J. Alzheimers Dis. 2010, 19, 1303–1315. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H. Characteristics of a SAM breeding colony at Japan SLC, Inc. In The Senescence-Accelerated Mouse (SAM): Achievement and Future Directions; Takada, T., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 15–23. [Google Scholar]
- Unno, K.; Yamamoto, H.; Toda, M.; Hagiwara, S.; Iguchi, K.; Hoshino, M.; Takabayashi, F.; Hasegawa-Ishii, S.; Shimada, A.; Hosokawa, M.; et al. Novel frame-shift mutation in Slc5a2 encoding SGLT2 in a strain of senescence-accelerated mouse SAMP10. Biochem. Biophys. Res. Commun. 2014, 454, 89–94. [Google Scholar] [CrossRef]
- Shimada, A.; Keino, H.; Satoh, M.; Kishikawa, M.; Seriu, N.; Hosokawa, M. Age-related progressive neuronal DNA damage associated with cerebral degeneration in a mouse model of accelerated senescence. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, B415–B421. [Google Scholar] [CrossRef] [Green Version]
- Shimada, A.; Keino, H.; Satoh, M.; Kishikawa, M.; Hosokawa, M. Age-related loss of synapses in the frontal cortex of SAMP10 mouse: A model of cerebral degeneration. Synapse 2003, 48, 198–204. [Google Scholar] [CrossRef]
- Shimada, A.; Keino, H.; Kawamura, N.; Chiba, Y.; Hosokawa, M. Limbic structures are prone to age-related impairments in proteasome activity and neuronal ubiquitinated inclusions in SAMP10 mouse: A model of cerebral degeneration. Neuropathol. Appl. Neurobiol. 2008, 34, 33–51. [Google Scholar] [CrossRef]
- Hasegawa-Ishii, S.; Takei, S.; Chiba, Y.; Furukawa, A.; Umegaki, H.; Iguchi, A.; Kawamura, N.; Yoshikawa, K.; Hosokawa, M.; Shimada, A. Morphological impairments in microglia precede age-related neuronal degeneration in senescence-accelerated mice. Neuropathology 2011, 31, 20–28. [Google Scholar] [CrossRef]
- Sasaki, T.; Unno, K.; Tahara, S.; Shimada, A.; Chiba, Y.; Hoshino, M.; Kaneko, T. Age-related increase of superoxide generation in the brains of mammals and birds. Aging Cell 2008, 7, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Unno, K.; Takabayashi, F.; Kishido, T.; Oku, N. Suppressive effect of green tea catechins on morphologic and functional regression of the brain in aged mice with accelerated senescence (SAMP10). Exp. Gerontol. 2004, 39, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Kishido, T.; Unno, K.; Yoshida, H.; Choba, D.; Fukutomi, R.; Asahina, S.; Iguchi, K.; Oku, N.; Hoshino, M. Decline in glutathione peroxidase activity is a reason for brain senescence: Consumption of green tea catechin prevents the decline in its activity and protein oxidative damage in ageing mouse brain. Biogerontology 2007, 8, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Unno, K.; Takabayashi, F.; Yoshida, H.; Choba, D.; Fukutomi, R.; Kikunaga, N.; Kishido, T.; Oku, N.; Hoshino, M. Daily consumption of green tea catechin delays memory regression in aged mice. Biogerontology 2007, 8, 89–95. [Google Scholar] [CrossRef]
- Unno, K.; Ishikawa, Y.; Takabayashi, F.; Sasaki, T.; Takamori, N.; Iguchi, K.; Hoshino, M. Daily ingestion of green tea catechins from adulthood suppressed brain dysfunction in aged mice. Biofactors 2008, 34, 263–271. [Google Scholar] [CrossRef]
- Unno, K.; Sugiura, M.; Ogawa, K.; Takabayashi, F.; Toda, M.; Sakuma, M.; Maeda, K.; Fujitani, K.; Miyazaki, H.; Yamamoto, H.; et al. Beta-cryptoxanthin, plentiful in Japanese mandarin orange, prevents age-related cognitive dysfunction and oxidative damage in senescence-accelerated mouse brain. Biol. Pharm. Bull. 2011, 34, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Unno, K.; Konishi, T.; Nakagawa, A.; Narita, Y.; Takabayashi, F.; Okamura, H.; Hara, A.; Yamamoto, H.; Iguchi, K.; Hoshino, M.; et al. Cognitive dysfunction and amyloid β accumulation are ameliorated by the ingestion of green soybean extract in aged mice. J. Funct. Foods 2015, 14, 345–353. [Google Scholar] [CrossRef] [Green Version]
- Shimoyoshi, S.; Takemoto, D.; Ono, Y.; Kitagawa, Y.; Shibata, H.; Tomono, S.; Unno, K.; Wakabayashi, K. Sesame Lignans Suppress Age-Related Cognitive Decline in Senescence-Accelerated Mice. Nutrients 2019, 11, 1582. [Google Scholar] [CrossRef] [Green Version]
- Pervin, M.; Unno, K.; Nakagawa, A.; Takahashi, Y.; Iguchi, K.; Yamamoto, H.; Hoshino, M.; Hara, A.; Takagaki, A.; Nanjo, F.; et al. Blood brain barrier permeability of (–)-epigallocatechin gallate, its proliferation-enhancing activity of human neuroblastoma SH-SY5Y cells, and its preventive effect on age-related cognitive dysfunction in mice. Biochem. Biophys. Rep. 2017, 9, 180–186. [Google Scholar] [CrossRef]
- Świątoniowska, N.; Sarzyńska, K.; Szymańska-Chabowska, A.; Jankowska-Polańska, B. The role of education in type 2 diabetes treatment. Diabetes Res. Clin. Pract. 2019, 151, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Ottosson-Laakso, E.; Tuomi, T.; Forsén, B.; Gullström, M.; Groop, P.H.; Groop, L.; Vikman, P. Influence of Familial Renal Glycosuria Due to Mutations in the SLC5A2 Gene on Changes in Glucose Tolerance over Time. PLoS ONE 2016, 11, e0146114. [Google Scholar] [CrossRef] [PubMed]
- Chiba, Y.; Sugiyama, Y.; Nishi, N.; Nonaka, W.; Murakami, R.; Ueno, M. Sodium/glucose cotranspoter 2 is expressed in choroid plexus epithelial cells and ependymal cells in human and mouse brains. Neuropathology 2020. [Google Scholar] [CrossRef] [PubMed]
- Needham, B.E.; Wlodek, M.E.; Ciccotosto, G.D.; Fam, B.C.; Masters, C.L.; Proietto, J.; Andrikopoulos, S.; Cappai, R. Identification of the Alzheimer’s disease amyloid precursor protein (APP) and its homologue APLP2 as essential modulators of glucose and insulin homeostasis and growth. J. Pathol. 2008, 215, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Bayer, T.A.; Paliga, K.; Weggen, S.; Wiestler, O.D.; Beyreuther, K.; Multhaup, G. Amyloid precursor-like protein 1 accumulates in neuritic plaques in Alzheimer’s disease. Acta Neuropathol. 1997, 94, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.R.; Urbanska, M.; Gozdz, A.; Swiech, L.J.; Nagalski, A.; Perycz, M.; Blazejczyk, M.; Jaworski, J. Cyr61, a matricellular protein, is needed for dendritic arborization of hippocampal neurons. J. Biol. Chem. 2013, 288, 8544–8559. [Google Scholar] [CrossRef] [Green Version]
- Li, X.C.; Jin, F.; Wang, B.Y.; Yin, X.J.; Hong, W.; Tian, F.J. The m6A demethylase ALKBH5 controls trophoblast invasion at the maternal-fetal interface by regulating the stability of CYR61 mRNA. Theranostics 2019, 9, 3853–3865. [Google Scholar] [CrossRef]
- Liang, Z.; Zhan, Y.; Shen, Y.; Wong, C.C.; Yates, J.R., 3rd; Plattner, F.; Lai, K.O.; Ip, N.Y. The pseudokinase CaMKv is required for the activity-dependent maintenance of dendritic spines. Nat. Commun. 2016, 7, 13282. [Google Scholar] [CrossRef] [Green Version]
- Shah, K.; Rossie, S. Tale of the Good and the Bad Cdk5: Remodeling of the Actin Cytoskeleton in the Brain. Mol. Neurobiol. 2018, 55, 3426–3438. [Google Scholar] [CrossRef]
- Inoue, T.; Ota, M.; Ogawa, M.; Mikoshiba, K.; Aruga, J. Zic1 and Zic3 regulate medial forebrain development through expansion of neuronal progenitors. J. Neurosci. 2007, 27, 5461–5473. [Google Scholar] [CrossRef]
- Irani, B.G.; Donato, J.J.; Olson, D.P.; Lowell, B.B.; Sacktor, T.C.; Reyland, M.E.; Tolson, K.P.; Zinn, A.R.; Ueta, Y.; Sakata, I.; et al. Distribution and neurochemical characterization of protein kinase C-theta and -delta in the rodent hypothalamus. Neuroscience 2010, 170, 1065–1079. [Google Scholar] [CrossRef] [Green Version]
- Graveling, A.J.; Frier, B.M. The risks of nocturnal hypoglycaemia in insulin-treated diabetes. Diabetes Res. Clin. Pract. 2017, 133, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Ebadi, S.A.; Darvish, P.; Fard, A.J.; Lima, B.S.; Ahangar, O.G. Hypoglycemia and cognitive function in diabetic patients. Diabetes Metab. Syndr. 2018, 12, 893–896. [Google Scholar] [CrossRef] [PubMed]
- Schilling, S.; Mehr, A.; Ludewig, S.; Stephan, J.; Zimmermann, M.; August, A.; Strecker, P.; Korte, M.; Koo, E.H.; Müller, U.C.; et al. APLP1 is a synaptic cell adhesion molecule, supporting maintenance of dendritic spines and basal synaptic transmission. J. Neurosci. 2017, 37, 5345–5365. [Google Scholar] [CrossRef]
- Duncan, L.E.; Cooper, B.N.; Shen, H. Robust findings from 25 years of PTSD genetics research. Psychiatry Rep. 2018, 20, 119. [Google Scholar]
- Shimada, A.; Tsuzuki, M.; Keino, H.; Satoh, M.; Chiba, Y.; Saitoh, Y.; Hosokawa, M. Apical vulnerability to dendritic retraction in prefrontal neurons of ageing SAMP10 mouse: A model of cerebral degeneration. Neuropathol. Appl. Neurobiol. 2006, 32, 1–14. [Google Scholar] [CrossRef]
- Miyamoto, M.; Kiyota, Y.; Yamazaki, N.; Nagaoka, A.; Matsuo, T.; Nagawa, Y.; Takeda, T. Age-related changes in learning and memory in the senescence-accelerated mouse (SAM). Physiol. Behav. 1986, 38, 399–406. [Google Scholar] [CrossRef]
- Konishi, T. Principal component analysis for designed experiments. BMC Bioinform. 2015, 16, S7. [Google Scholar] [CrossRef] [Green Version]
- Konishi, T. Three-parameter lognormal distribution ubiquitously found in cDNA microarray data and its application to parametric data treatment. BMC Bioinform. 2004, 5, 5. [Google Scholar]
- Khan, Q.E.; Press, C.M.; Sehic, A.; Landin, M.A.; Risnes, S.; Osmundsen, H. Expression of prion gene and presence of prion protein during development of mouse molar tooth germ. Eur. J. Oral Sci. 2010, 118, 559–565. [Google Scholar] [CrossRef]
- Delépine, C.; Nectoux, J.; Letourneur, F.; Baud, V.; Chelly, J.; Billuart, P.; Bienvenu, T. Astrocyte transcriptome from the Mecp2(308)-truncated mouse model of Rett Syndrome. Neuromol. Med. 2015, 17, 353–363. [Google Scholar] [CrossRef]
- Dong, W.; Yang, W.; Li, F.; Guo, W.; Qian, C.; Wang, F.; Li, C.; Lin, L.; Lin, R. Electroacupuncture improves synaptic function in SAMP8 mice probably via inhibition of the AMPK/eEF2K/eEF2 signaling pathway. Evid. Based Complement. Alternat. Med. 2019, 2019, 8260815. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Symbol | Full Name | ΔZ | p | |
|---|---|---|---|---|
| Down-Regulated | Aplp1 | amyloid beta (A4) precursor-like protein 1 | −1.1688 | 6.77 × 10−48 |
| Olfr716 | olfactory receptor 716 | −0.4277 | 0.0013 | |
| Trav14-1 | T cell receptor alpha variable 14-1 | −0.5237 | 0.0031 | |
| Cyr61 | cysteine rich protein 61 | −0.2115 | 0.0004 | |
| Ifna12 | interferon alpha 12 | −0.3784 | 0.0004 | |
| Sult2a2 | sulfotransferase family 2A, dehydroepiandrosterone (DHEA)-preferring, member 2 | −0.3743 | 0.0072 | |
| Pth | parathyroid hormone | −0.2515 | 0.0014 | |
| LOC100043315 | uncharacterized LOC100043315 | −0.2768 | 0.0087 | |
| Rpl28-ps4 | ribosomal protein L28, pseudogene 4 | −0.2998 | 0.0024 | |
| Prl2c1 | Prolactin family 2, subfamily c, member 1 | −0.2691 | 0.0082 | |
| Up-Regulated | Camkv | CaM kinase-like vesicle-associated | 1.5327 | 6.73 × 10−47 |
| Mir148b | microRNA 148b | 0.4986 | 0.0003 | |
| Vmn1r177 | vomeronasal 1 receptor 177 | 0.3498 | 0.0078 | |
| Zic1 | zinc finger protein of the cerebellum 1 | 0.3551 | 2.67 × 10−16 | |
| LOC434035 | immunoglobulin kappa-chain VK-1 | 0.3064 | 0.0069 | |
| Prkcd | protein kinase C, delta | 0.3021 | 1.93 × 10−12 | |
| Aspn | asporin | 0.2295 | 0.0052 | |
| Vmn1r8 | vomeronasal 1 receptor 8 | 0.3163 | 0.0053 | |
| Tcf7l2 | transcription factor 7 like 2, T cell specific, HMG box | 0.2341 | 0.0002 | |
| Calb2 | calbindin 2 | 0.2799 | 4.73 × 10−8 | |
| ΔZ = expression level (SAMP10-∆Sglt2 − SAMP10(+)) | ||||
| Mouse Line | SAMR1 | SAMP10-ΔSglt2 | SAMP10(+) |
|---|---|---|---|
| Lifespan | Long | Short | Short |
| Cerebral atrophy | − | + | + |
| Depression | − | + | + |
| Mutation in SGLT2 | − | + | − |
| Glucose in urine | − | + | − |
| Glucose in blood | Normal | Low in young | Normal |
| Memory retention | High | Low in aged | High |
| Aplp1 in the hippocampus | Normal | Low in young | Normal in young |
| Camkv in the hippocampus | Normal | Slightly high | Low |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unno, K.; Takagi, Y.; Konishi, T.; Suzuki, M.; Miyake, A.; Kurotaki, T.; Hase, T.; Meguro, S.; Shimada, A.; Hasegawa-Ishii, S.; et al. Mutation in Sodium-Glucose Cotransporter 2 Results in Down-Regulation of Amyloid Beta (A4) Precursor-Like Protein 1 in Young Age, Which May Lead to Poor Memory Retention in Old Age. Int. J. Mol. Sci. 2020, 21, 5579. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21155579
Unno K, Takagi Y, Konishi T, Suzuki M, Miyake A, Kurotaki T, Hase T, Meguro S, Shimada A, Hasegawa-Ishii S, et al. Mutation in Sodium-Glucose Cotransporter 2 Results in Down-Regulation of Amyloid Beta (A4) Precursor-Like Protein 1 in Young Age, Which May Lead to Poor Memory Retention in Old Age. International Journal of Molecular Sciences. 2020; 21(15):5579. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21155579
Chicago/Turabian StyleUnno, Keiko, Yoshiichi Takagi, Tomokazu Konishi, Mitsuhiro Suzuki, Akiyuki Miyake, Takumi Kurotaki, Tadashi Hase, Shinichi Meguro, Atsuyoshi Shimada, Sanae Hasegawa-Ishii, and et al. 2020. "Mutation in Sodium-Glucose Cotransporter 2 Results in Down-Regulation of Amyloid Beta (A4) Precursor-Like Protein 1 in Young Age, Which May Lead to Poor Memory Retention in Old Age" International Journal of Molecular Sciences 21, no. 15: 5579. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms21155579